Prostate MRI
The interpretation of Prostate MRI is a complex and constantly evolving topic. References are provided in the didactic text preceding and following the case examples. Case examples have been selected to summarize important teaching points.
Authors: Ravi V. Gottumukkala, MD, Leslie K.Lee, MD
Date: June 30, 2021
PI-RADS v2.1 (https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2-1.pdf?la=en) was introduced in 2019, and at the time of writing is the most current version.
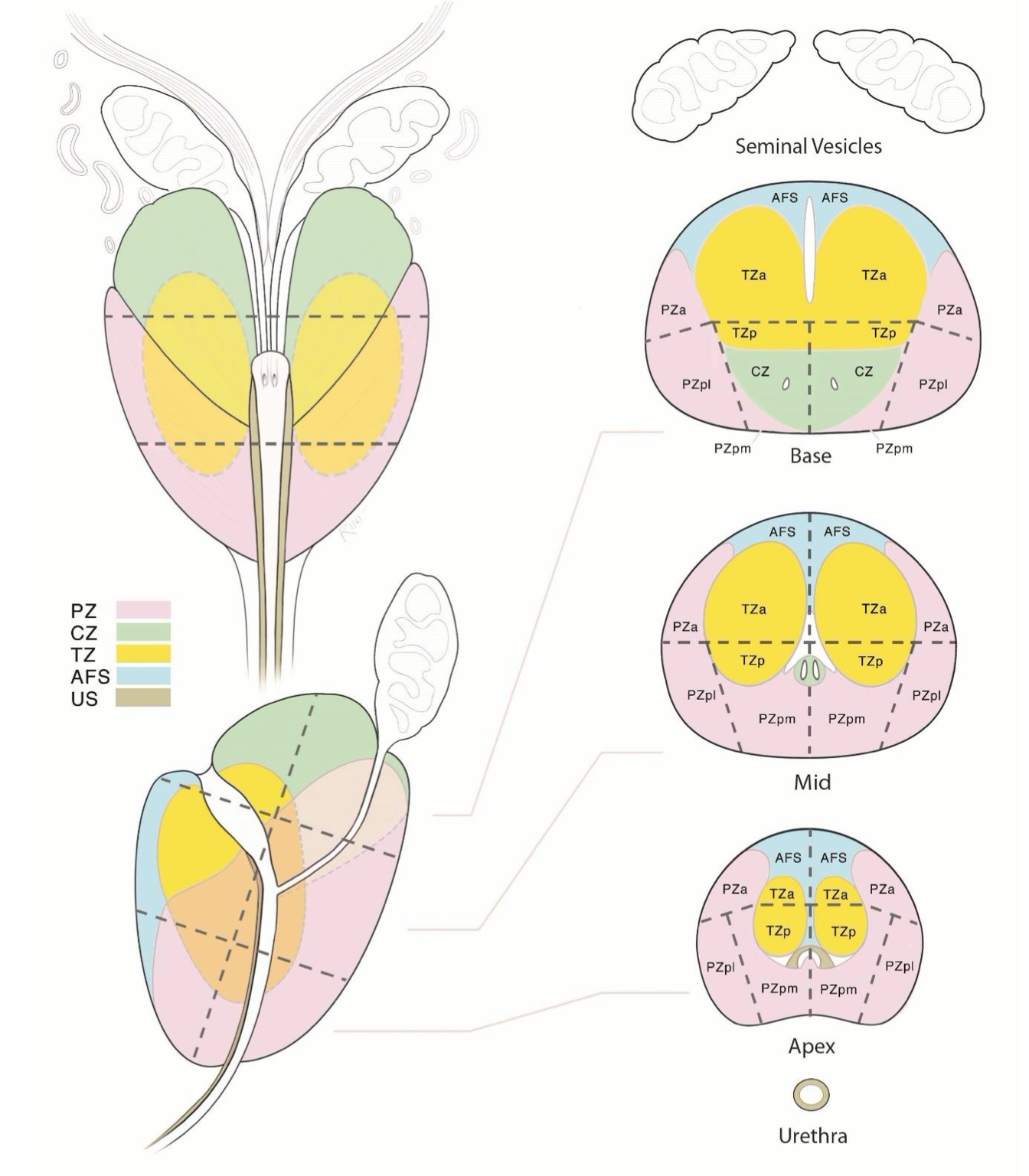
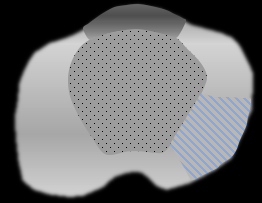
Anatomical Zones of the Prostate Gland as seen on MRI2
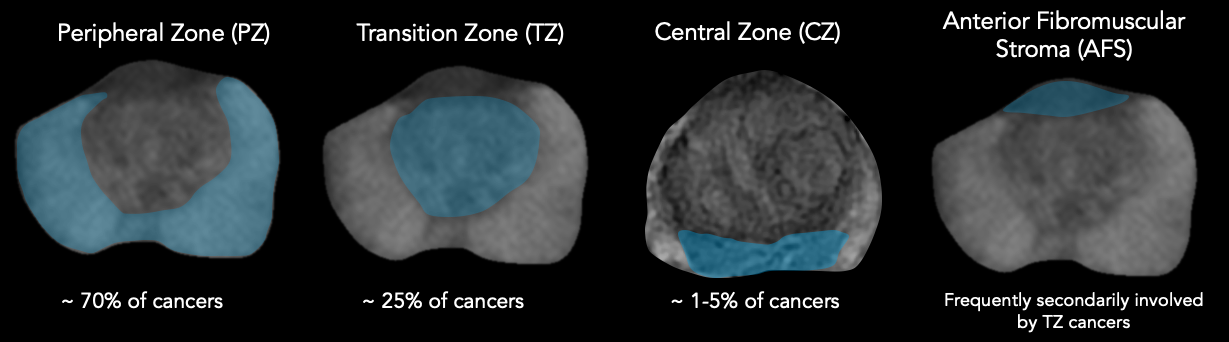
Sector Map
https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/PI-RADS
Implication of PI-RADS Assessment Scores
| Score | Criteria |
|---|---|
| 1 | Clinically significant cancer highly unlikely |
| 2 | Clinically significant cancer unlikely |
| 3 | Clinically significant cancer equivocal |
| 4 | Clinically significant cancer likely |
| 5 | Clinically significant cancer highly likely |
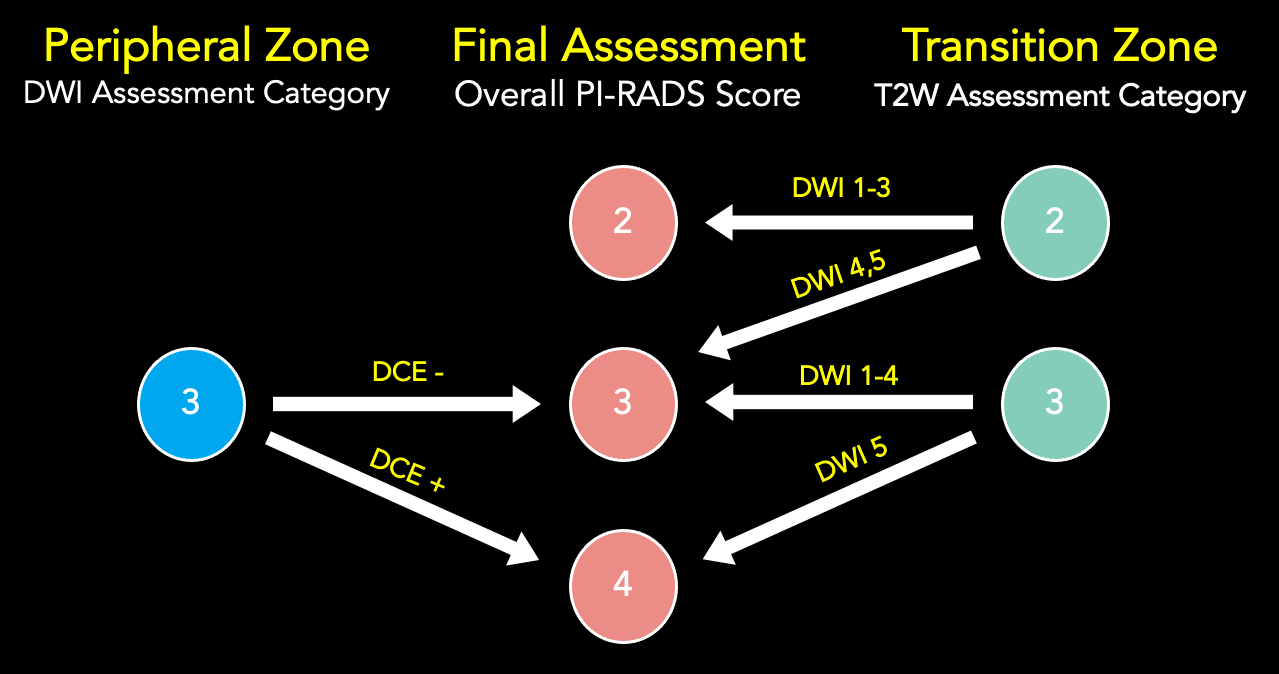
Basic Scoring approach in Each Zone
Derived from PI-RADS V2.1 manual pages 11-141; specific scoring criteria for the PZ and TZ are included in the dedicated sections later in this document
- Peripheral Zone – DWI is the primary sequence for scoring; DCE relevant for DWI score of 3
- Transition Zone – T2W is the primary sequence for scoring; DWI relevant for T2W scores of 2 or 3
- Central Zone – No discrete scoring criteria, assess for focal early enhancement and/or asymmetry on DWI and T2W
- AFS – Apply TZ or PZ scoring criteria depending on perceived most likely zone of lesion origin
Specific Situations: Category Upgrades
PI-RADS v2.1 Scoring: DWI for Peripheral Zone or Transition Zone
| Score | Criteria |
| 1 | No DWI/ADC abnormality |
| 2 | Linear/wedge shaped hypointense on ADC and/or linear/wedge shaped hyperintense on high b-value DWI |
| 3 | Focal (discrete and different from the background) hypointense on ADC and/or focal hyperintense on high b-value DWI; may be markedly hypointense on ADC or markedly hyperintense on high b-value DWI, but not both. |
| 4 | Focal markedly hypointense on ADC and markedly hyperintense on high b-value DWI; <1.5cm in greatest dimension |
| 5 | Same as 4 but ≥1.5cm in greatest dimension or definite extraprostatic extension/invasive behavior |
DWI assessment should occur on the “high” b-value images (b ≥ 1400 s/mm2)
Adapted from the PI-RADS V2.1 Manual1
PI-RADS v2.1 Scoring: DCE for Peripheral Zone
| Score | Criteria |
| Negative |
ANY of the following:
|
| Positive |
Must have ALL of the following features:
|
Scrolling through both the raw DCE and subtraction DCE images is helpful
Adapted from the PI-RADS V2.1 Manual1
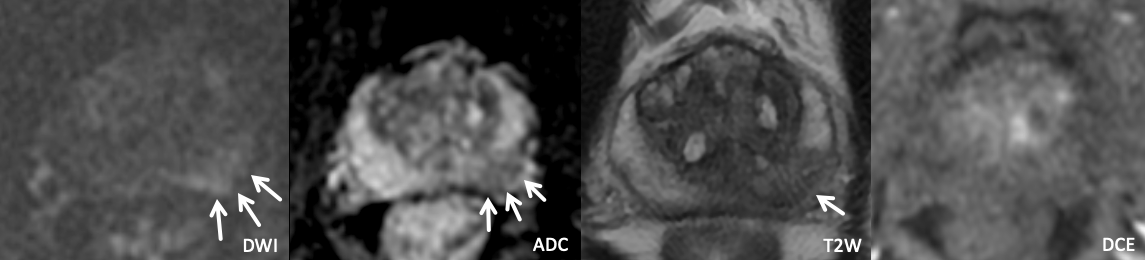
Example 1 – What PI-RADS score would you assign to this lesion?
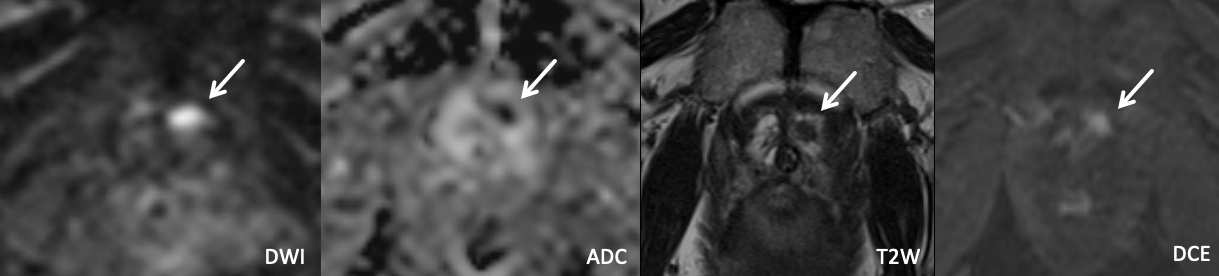
|
Findings: Lesion in the left anterior apical peripheral zone with marked DWI hyperintensity, marked ADC hypointensity, homogeneous circumscribed T2 hypointensity, and positive DCE.
|
Example 2 – What PI-RADS score would you assign to this lesion?
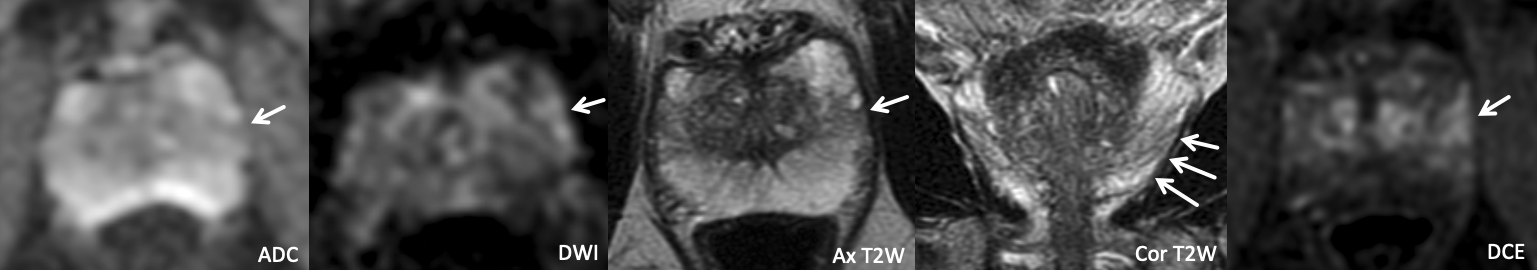
|
Findings: Lesion in the left anterior mid peripheral zone with mild linear ADC hypointensity, mild/moderate wedge-shaped DWI hyperintensity, wedge-shaped T2W hypointensity with striated configuration particularly in the coronal plane, and positive DCE.
|
Example 3 – What PI-RADS score would you assign to this lesion?
|
Findings: Lesion in the left posteromedial/posterolateral basal peripheral zone with mild DWI hyperintensity, mild ADC hypointensity, homogeneous T2 hypointensity, and negative DCE. |
Example 4 – What PI-RADS score would you assign to this lesion?
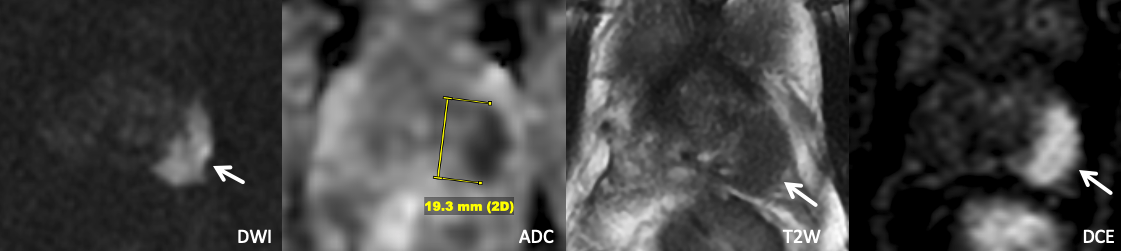
|
Findings: 1.9 cm in the left anterior/posterolateral mid peripheral zone with marked DWI hyperintensity, marked ADC hypointensity, homogeneous T2 hypointensity, and positive DCE. |
Example 5 – What PI-RADS score would you assign to this lesion?
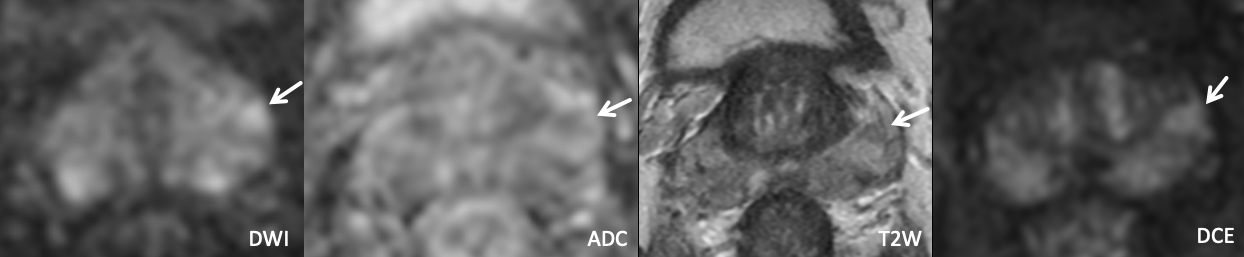
|
Findings: Lesion in the left anterior basal peripheral zone with mild/moderate DWI hyperintensity, mild/moderate ADC hypointensity, homogenous T2 hypointensity (equivocally but not definitely wedge-shaped), and positive DCE. |
Benign Peripheral Zone Abnormalities
Prostatitis, Post-inflammatory Scar, and Focal Prostate Atrophy
- Clarification of clinical and radiologic terminology
- Clinically, the term “prostatitis” signifies either acute or chronic infection/inflammation of the prostate gland, sometimes with associated pain, recurrent UTI, and/or fluctuating PSA3
- As opposed to clinically defined prostatitis, the MRI finding commonly reported as “prostatitis” or “sequelae of prostatitis” most often reflects areas of parenchymal signal abnormality related to4:
- Post-inflammatory scar from prior prostatitis, often subclinical
- Active acute or chronic prostatitis
- Focal prostate atrophy, a common benign pathologic finding sometimes associated with inflammation
- Imaging features of post-inflammatory/inflammatory lesions4–6
- Range of appearances from characteristically benign to indistinguishable from cancer, typically with some degree of signal abnormality on T2, DWI, and DCE.4–6
- Features more typical of post-inflammatory/inflammatory findings include: wedge-shaped/linear morphology (following the normal glandular ductal drainage patterns), patchy/ill-defined appearance, striated appearance, lobar or diffuse distribution (as opposed to a focal lesion), associated volume loss, and milder diffusion abnormality.4–6
- Equivocal cases may be accompanied by a PI-RADS assessment.
- BCG-related granulomatous prostatitis
- Can produce diffuse or focal signal abnormalities that are sometimes indistinguishable from cancer
- Typical appearance includes T2 hypointensity and ADC hypointensity, with either isointensensity or hyperintensity on DWI7
- Rim-enhancement with central hypoenhancement, when present, is characteristic and may suggest the diagnosis in the context of prior BCG therapy.8
- When suspected, options for further assessment include biopsy for confirmation or short interval follow-up MRI after antibiotic therapy.
| Typical Morphologic Features of Sequelae of Prostatitis |
 |
| Wedge-shaped/linear morphology, striated appearance on T2W images, regional/lobar distribution, volume loss |
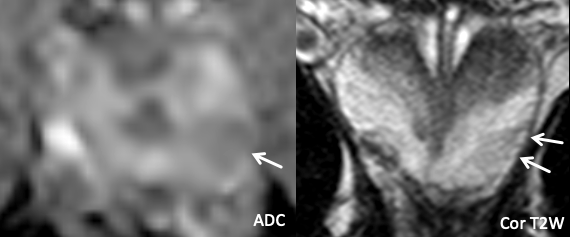
| Example 1 | Example 2 |
 |
 |
| Imaging Findings: Lesion in the left posterolateral mid-apical peripheral zone with mild wedge-shaped restricted diffusion, and a striated appearance on T2-weighed images. PI-RADS 2. Final Diagnosis:Sequelae of prostatitis |
Imaging Findings: Lesion in the right posterolateral mid peripheral zone with marked restricted diffusion, homogeneous T2 hypointensity in a lobar distribution, and positive DCE. PI-RADS 5 by imaging criteria. Pathology (targeted biopsy): Benign prostate tissue. Final Diagnosis: Likely sequelae of prostatitis |
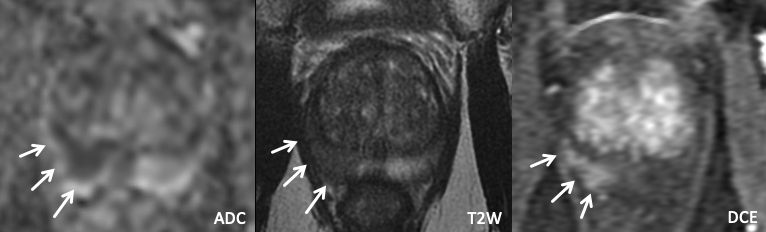
| Example 3 | Example 4 |
 |
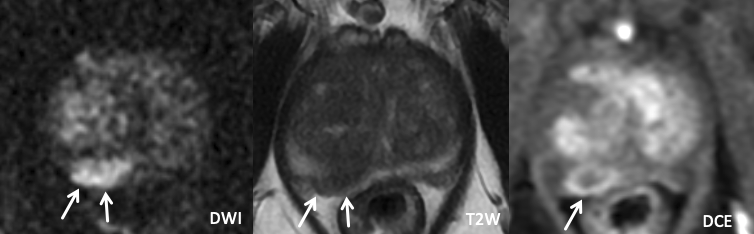 |
| Imaging Findings: Lesion in the left posterolateral mid peripheral zone with moderate restricted diffusion, suggestion of wedge-shaped T2W hypointensity, and positive DCE. Note the associated volume loss compared to the contralateral PZ. Appearance may represent prostatitis though biopsy was performed for confirmation. Pathology (targeted biopsy): Benign prostate tissue. Final Diagnosis: Sequelae of prostatitis |
Imaging Findings: Lesion in the right posteromedial/posterolateral mid peripheral zone with marked restricted diffusion, homogeneous T2 hypointensity, and rim-enhancement on DCE. PI-RADS 4 by imaging criteria though likely BCG granuloma given rim-enhancement and hx of BCG therapy. Pathology/Final Diagnosis: BCG-related granulomatous prostatitis. |
Extruded/Ectopic BPH Nodules
- While BPH largely arises in the TZ, nodules may less commonly either protrude exophytically into the PZ or arise ectopically in the PZ; in practice, both of these processes are often referred to as “extruded BPH”1,5,9.
- Imaging features of extruded BPH: similar to BPH in the TZ, appearance may span the range from typical (circumscribed, heterogeneous, encapsulated) to atypical (homogeneous and non-encapsulated) BPH, the latter of which may be indistinguishable from cancer when in the PZ1,5,9.
- Categorized as PI-RADS 2 when outside the TZ but have characteristic imaging findings of BPH1
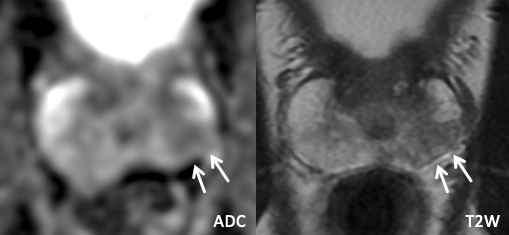
| Example of Extruded BPH |
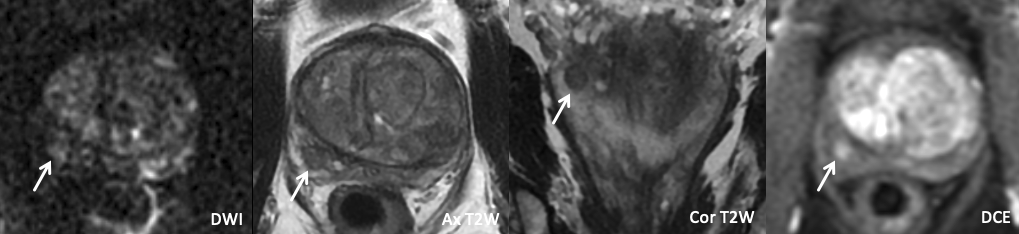 |
| Imaging Findings: Lesion in the right posterolateral base peripheral zone with mild/moderate restricted diffusion, circumscribed heterogeneous T2W signal with encapsulation, and positive DCE. PI-RADS 2 (Extruded BPH). |
Post-biopsy Hemorrhage
- T1-hyperintense signal (as seen on pre-Gad) that may persist for several months after biopsy (due to anticoagulant effect of citrate produced by normal prostate).
- Interface with tumor visualization4,5,9
- Tumor may be less conspicuous on a background of hemorrhage-related T2 hypointense signal.
- Intrinsic T1 hyperintensity obscures assessment for enhancement on DCE, and subtraction images (post-Gad minus pre-Gad) must be carefully reviewed.
- ADC map can identify areas of marked diffusion restriction suspicious for tumor, while hemorrhage is typically either non-restricting or mildly restricting
- Hemorrhage exclusion sign: Areas of tumor are commonly spared from hemorrhage-related intrinsic T1 hyperintensity, which may facilitate lesion delineation
| Example of Post-biopsy Hemorrhage (with “hemorrhage exclusion sign”) |
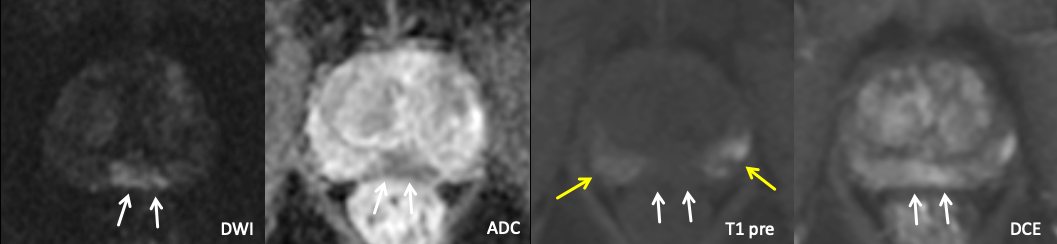 |
| Markedly diffusion restricting lesion involving both the bilateral posteromedial mid peripheral zones, positive for cancer on recent biopsy. Note intrinsic T1 hyperintense hemorrhage in the peripheral zones bilaterally (yellow arrows), which spares the area of the lesion. The lesion enhanced avidly on DCE. |
PI-RADS v2.1 Scoring: T2W for Transition Zone
| Score | Criteria |
| 1 | Normal appearing TZ (rare) or a round, completely encapsulated nodule. (“typical nodule”) |
| 2 | A mostly encapsulated nodule OR a homogeneous circumscribed nodule without encapsulation. (“atypical nodule”) OR a homogeneous mildly hypointense area between nodules |
| 3 | Heterogeneous signal intensity with obscured margins. Includes others that do not qualify as 2, 4, or 5 |
| 4 | Lenticular or non-circumscribed, homogeneous, moderately hypointense, and <1.5 cm in greatest dimension |
| 5 | Same as 4, but ≥1.5cm in greatest dimension or definite extraprostatic extension/invasive behavior |
Adapted from the PI-RADS V2.1 Manual
PI-RADS v2.1 Scoring: DWI for Transition Zone or Peripheral Zone
| Score | Criteria |
| 1 | No DWI/ADC abnormality |
| 2 | Linear/wedge shaped hypointense on ADC and/or linear/wedge shaped hyperintense on high b-value DWI |
| 3 | Focal (discrete and different from the background) hypointense on ADC and/or focal hyperintense on high b-value DWI; may be markedly hypointense on ADC or markedly hyperintense on high b-value DWI, but not both. |
| 4 | Focal markedly hypointense on ADC and markedly hyperintense on high b-value DWI; <1.5cm in greatest dimension |
| 5 | Same as 4 but ≥1.5cm in greatest dimension or definite extraprostatic extension/invasive behavior |
Adapted from the PI-RADS V2.1 Manual
How do Transition zone adenocarcinomas differ from Peripheral Zone Cancers?
- Biology
- Tend to be lower grade and more likely organ-confined than PZ cancer, though larger in size on average10
- Have a higher predilection for being located anterior and inferior in the gland, with tendency for early extension into the anterior fibromuscular stroma11–13
- Imaging detection10–14
- MRI has lower accuracy and higher interobserver variability for TZ compared to PZ cancers, largely owing to the confounding effect of BPH12
- Lesion morphology and texture on T2W imaging are the primary factors used for PI-RADS categorization, with features more typical of cancer including: Lenticular shape, non-circumscribed margins, and/or homogeneous, moderate T2W hypointensity.
- Stromal BPH can demonstrate both marked restricted diffusion and early intense enhancement on DCE similar to cancer. When T2W morphology is equivocal, disproportionality of diffusion restriction and enhancement within a lesion compared to neighboring BPH can help raise suspicion.
- Both DWI and DCE are very helpful for improving lesion conspicuity and prompting further interrogation on T2W images, and DWI has been shown to improve sensitivity for cancer detection in the TZ compared to T2W alone14.
- Tissue diagnosis
- TZ lesions can be challenging to detect by transrectal biopsy or physical exam, given anterior predilection; therefore, MRI is crucial in directing targeted biopsy, and the anterior TZ deserves scrutiny in cases with negative prior biopsy but rising PSA12.
- Very anterior and apical lesions are typically easier to target by MRI-guided transperineal biopsy, which can be considered based on MRI findings and/or prior negative transrectal biopsy15.
Example 1 – What PI-RADS score would you assign to this lesion?
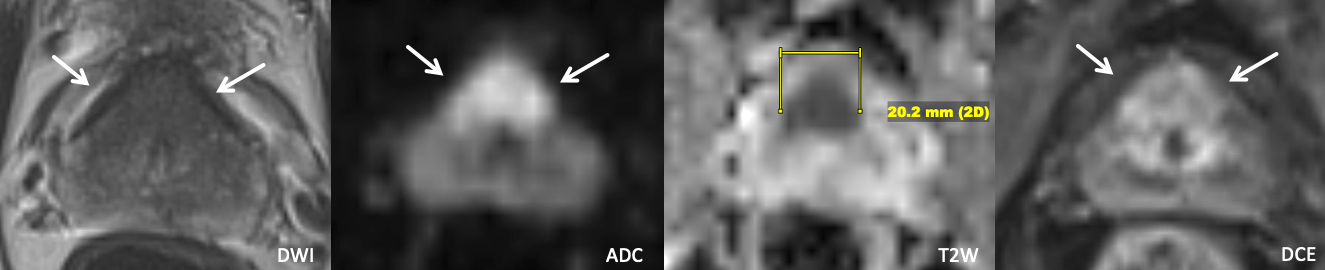
|
Findings: 2.0 cm lesion centered in the anterior transition zone bilaterally with ill-defined homogeneous T2 hypointensity, with marked restricted diffusion and enhancement disproportionate to the neighboring TZ. The lesion replaces the expected location of the anterior fibromuscular stroma, which not well visualized.
|
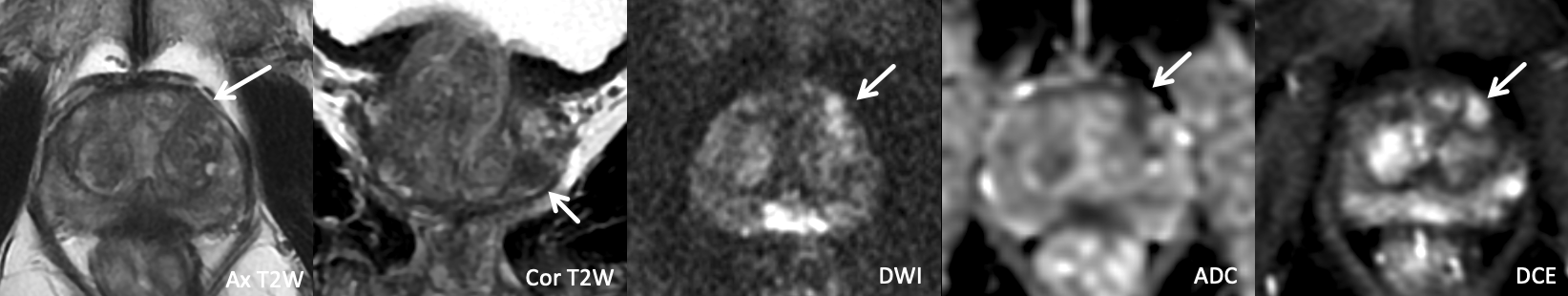
Example 2 – What PI-RADS score would you assign to this lesion?
|
Findings: Lesion in the right anterior mid transition zone that demonstrates heterogeneous T2W signal with encapsulation, marked restricted diffusion, and positive DCE.
|
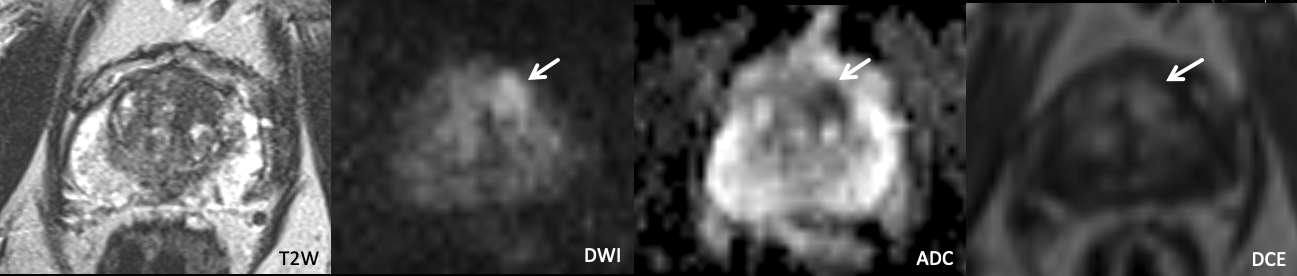
Example 3 – What PI-RADS score would you assign to this lesion?
|
Findings: Lesion in the left anterior apical transition zone with ill-defined homogeneous T2 hypointensity, marked restricted diffusion, and positive DCE in portions of the lesion.
|
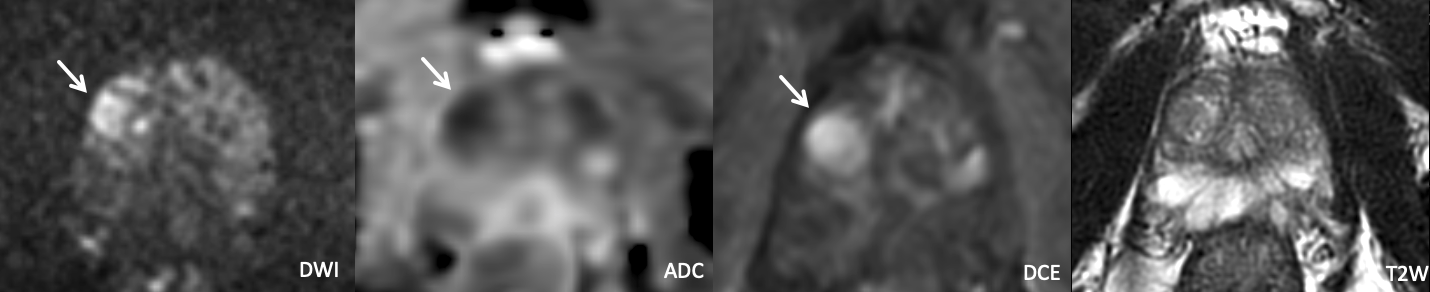
Example 4 – What PI-RADS score would you assign to this lesion?
|
Findings: Lesion in the left anterior mid peripheral zone with relatively homogeneous T2 hypointensity that in the coronal plane appears circumscribed but not clearly encapsulated. The lesion markedly restricts diffusion and is DCE positive. A separate lesion is also seen in the bilateral posteromedial peripheral zones, disregarded for the purposes of this teaching case.
|
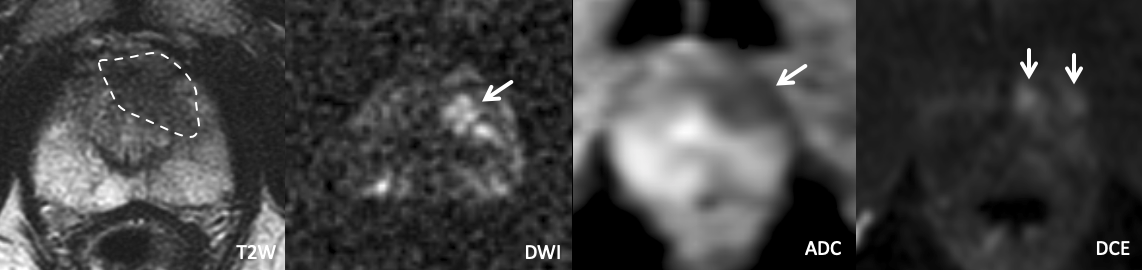
Example 5 – What PI-RADS score would you assign to this lesion?
|
Findings: The T2W appearance of the TZ is not particularly remarkable at first glance. However, a focal area of marked restricted diffusion and positive DCE in the left anterior mid transition zone corresponds to an area of ill-defined T2 hypointensity.
|
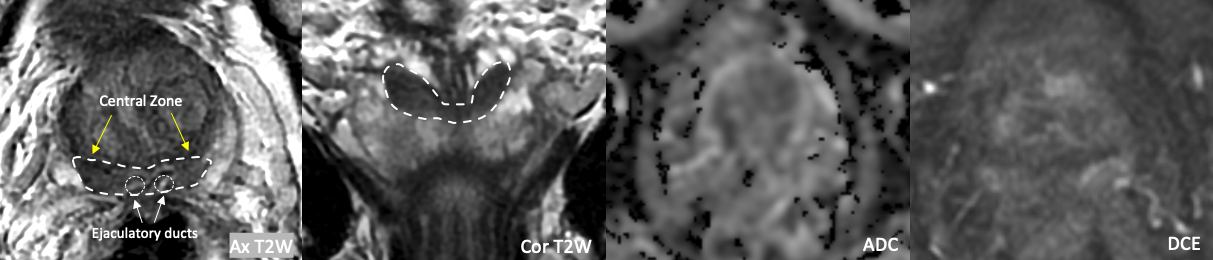
What is the central zone?1,4,5,9,16
- T2 hypointense tissue surrounding the ejaculatory ducts at the prostatic base that atrophies and becomes less conspicuous with age
- Normal CZ can sometimes demonstrate T2W and DWI characteristics similar to cancer, though should not demonstrate early enhancement
- Cancers rarely originate in the CZ (1-5%), and much more often involve it secondarily from the adjacent PZ or TZ
How do you score central zone lesions in PI-RADS?1
- PI-RADS v2.1 does not stipulate specific criteria for scoring CZ lesions, but rather suggests that focal early enhancement and/or asymmetry on DWI and T2W images may indicate the presence of PCa
- If a cancer secondarily involves the CZ, scoring can applied to the zone from which the lesion most likely arises
Example 1 – Normal Central Zone
|
Teaching points:
|
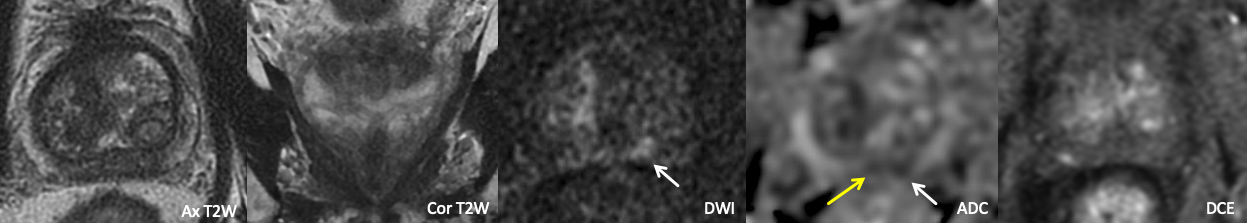
Example 2a – How would you characterize this finding?
|
Findings: There is moderate asymmetric restricted diffusion within the left CZ, though the contralateral CZ demonstrates a similar degree of ADC hypointensity (yellow arrow) over a smaller area. The T2W images are unremarkable, and DCE is negative.
|
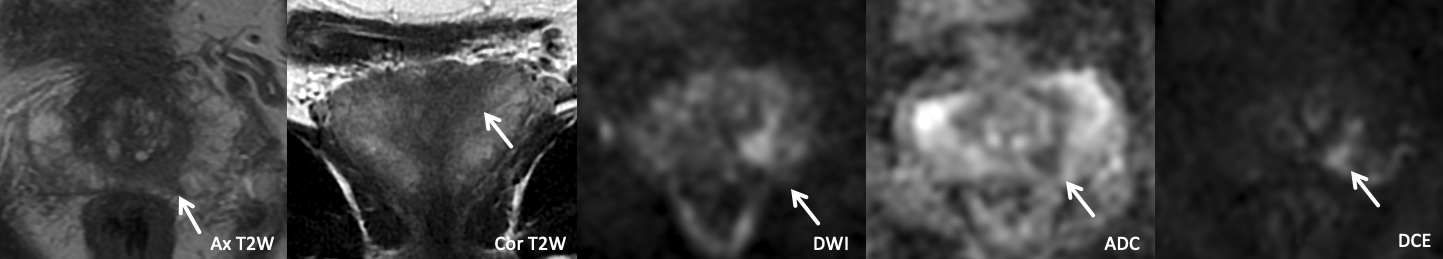
Example 2b – How would you categorize this finding?
|
Findings: There is marked asymmetric restricted diffusion within the left CZ, with corresponding strikingly asymmetric T2 hypointensity and intense, early, focal, asymmetric enhancement on DCE. |
Extraprostatic Extension (EPE)
Anatomic Principles
- Prostate does not actually have a true capsule, but the terms “capsule” or “outer prostate margin” are frequently used to refer to a condensed band of prostatic stroma along the gland periphery, sometimes visible as a thin T2 hypointense line17
- EPE = tumor beyond the prostatic capsule/margin
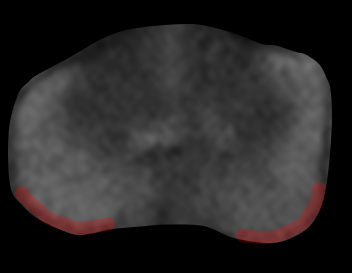
- Certain areas of the gland have a higher propensity for EPE due to anatomic factors (see table below); drawing attention to tumor contact in these locations is helpful, even if there is no frank EPE by imaging18,19
Locations with higher risk of EPE
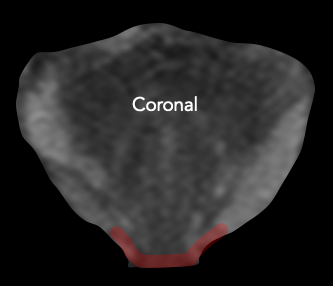
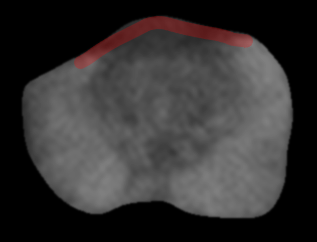
| Posterolateral base | Apex | Anterior fibromuscular stroma | Central zone and base of seminal vesicles |
 |
 |
 |
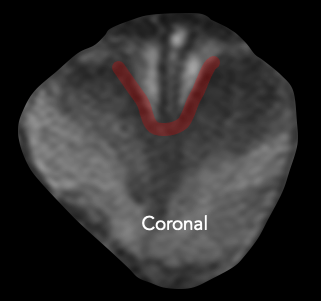 |
| Early perineural spread along penetrating branches from the neurovascular bundles | Capsule deficient in this location, facilitating spread into striated muscle of external sphincter | Capsule less defined in this location, facilitating anterior EPE of TZ cancers | No capsule surrounding the intraprostatic course of ejaculatory ducts or base of seminal vesicles, providing a route for EPE (including directly into the seminal vesicles) |
Imaging Assessment
- MRI has Se ~ 60% and Sp ~ 90% for EPE20.
- MRI plays a role in identifying definite extraprostatic extension or drawing attention to areas at risk of microscopic EPE, which may relate to surgical planning17.
- T2W images are often useful for assessing EPE, as they provide sharp depiction of the prostate border; DCE can also be a helpful adjunct.
- In the absence of frank EPE, one or more indirect signs (see table below) increases likelihood of EPE at pathology21, especially in an area of anatomic weakness.
| Direct Sign | Indirect Signs | |||
| Frank EPE: tumor extension beyond prostatic margin into periprostatic fat or adjacent structures |
Broad capsular contact: > 10-15 mm tumor contact with prostatic margin; sensitive but not specific |
Contour bulge: smooth convex protrusion of prostatic margin overlying tumor |
Contour irregularity |
Neurovascular bundle asymmetry |
 |
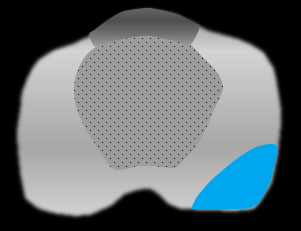 |
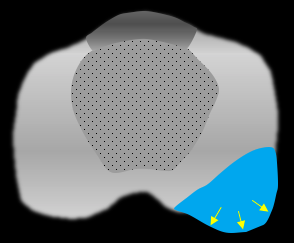 |
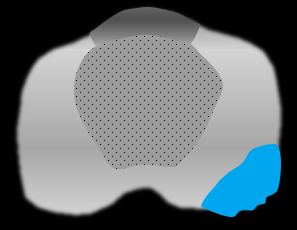 |
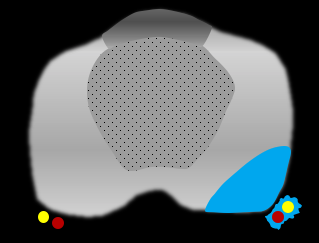 |
| Example 1 | >Example 2 |
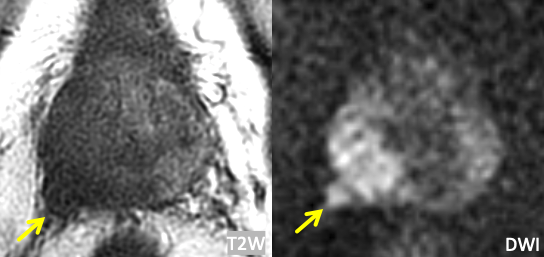 |
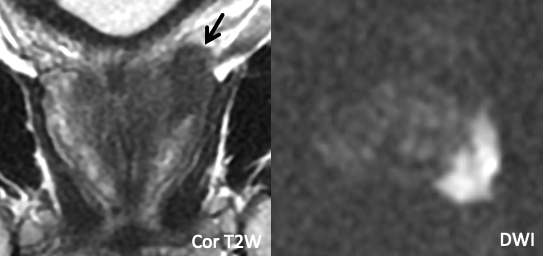 |
| Imaging Findings: Frank EPE at the right posterolateral base. | Imaging Findings: Frank EPE at the posterior base, best seen on coronal T2W images. Multiplanar assessment is helpful. |
| Example 3 | Example 4 |
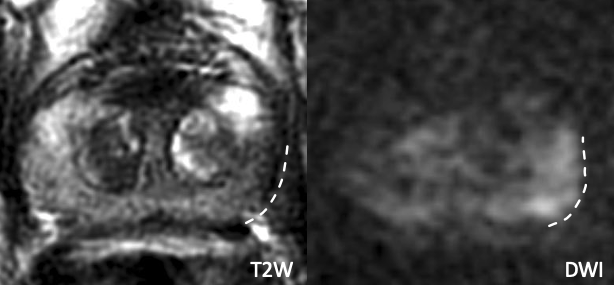 |
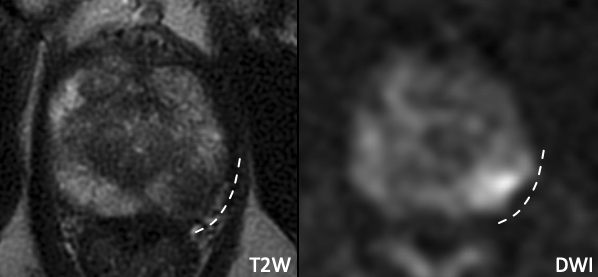 |
| Imaging Findings: Broad capsular contact at the posterolateral base without frank EPE. Pathology: Positive for EPE. |
Imaging Findings: Broad capsular contact at the posterolateral mid gland without frank EPE. Similar appearance as Example 3. Pathology: Negative for EPE |
| Example 5 | Example 6 |
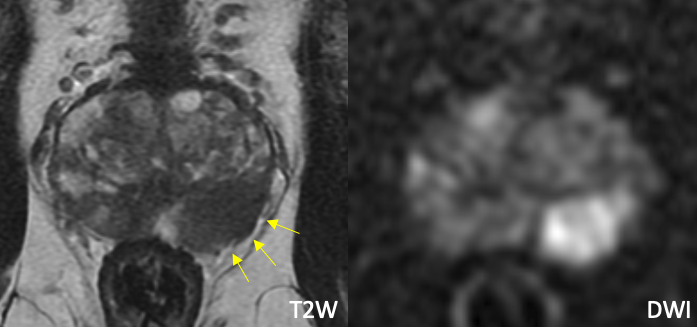 |
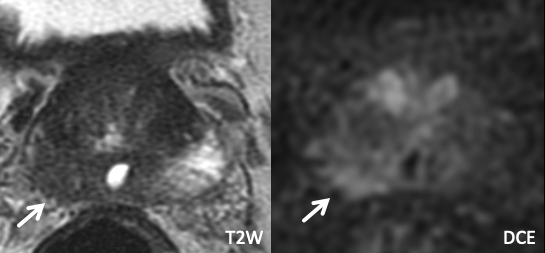 |
| Imaging Findings: Bulging of the prostate border and broad capsular contact along the left posterolateral mid to basal gland, without frank EPE. Based on these findings, left neurovascular bundle was removed at surgery. Pathology: Positive for EPE. |
Imaging Findings: Irregularity along the right posterolateral prostate border, evident both on T2W and DCE, without frank EPE. Based on these findings, right neurovascular bundle was removed at surgery. Pathology: Positive for EPE. |
Seminal Vesicle Invasion (SVI)
Mechanisms of Seminal Vesicle Invasion
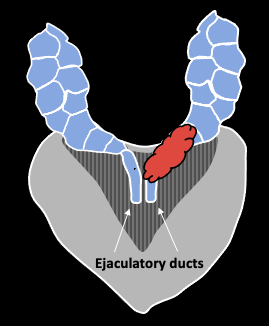 |
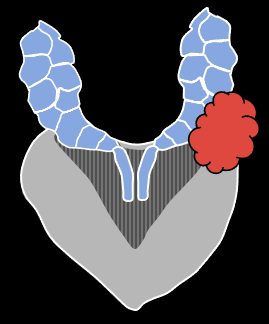 |
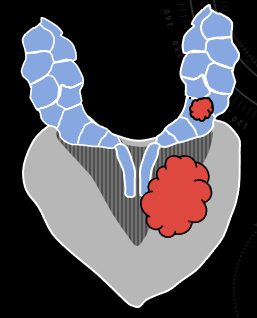 |
| Direct spread along ejaculatory duct into SV | EPE through adjacent capsule, with secondary invasion of SV | Discontinuous tumor deposit in SV (uncommon) |
Imaging Assessment
- MRI has Se ~ 60% and Sp ~ 95% for SVI20
- SVI manifests as focal or diffusely abnormal signal (T2 hypointensity, restricted diffusion, hyperenhancement) along the SV lumen or wall1.
- T2W imaging, particularly the coronal and sagittal planes, is often useful due to sharp anatomic definition. DWI and DCE are adjunctive.
- Scrutinize the base of the seminal vesicle for abutment even if there is no frank SVI, as this finding has an association with SVI at pathologic assessment22.
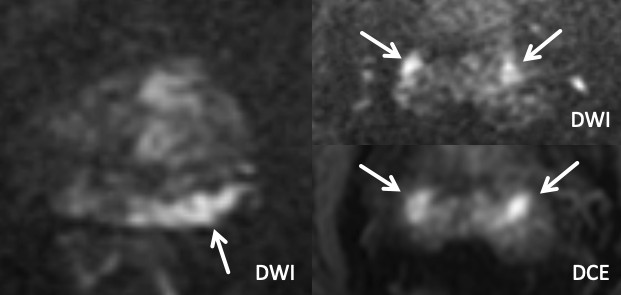
| Example 1 |
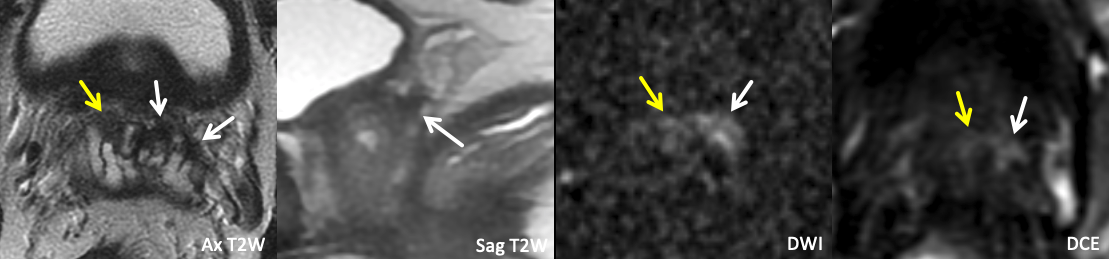 |
| Imaging Findings: Foci of T2 hypointensity, restricted diffusion, and enhancement in the caudal left SV (white arrows), and to a lesser extent in the right SV (yellow arrows). Note the T2 hypointense tumor signal clearly extending into the left SV on the Sag T2W images, with effacement of the normal tapered T2 hyperintense SV base. Findings indicate bilateral SVI, left greater than right. Path: Bilateral SVI |
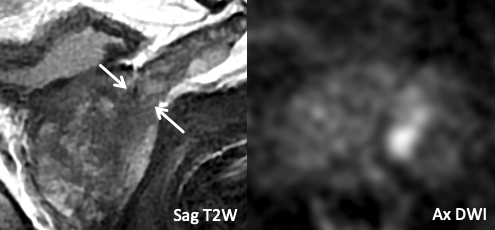
| Example 2 | Example 3 |
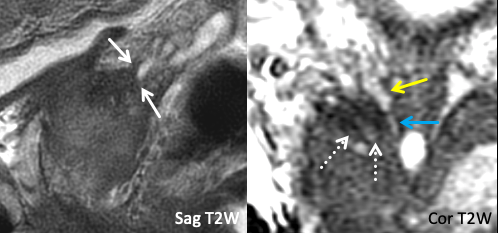
 |
 |
| Imaging Findings: T2-hypointense tumor signal abutment of the base of the left SV without frank SVI. Tapering of the SV base remains sharply defined. Multiplanar T2W assessment is helpful. Path: Abutment of SV base, without SVI. |
Imaging Findings: T2-hypointense tumor signal abuts of the base of the right SV without frank SVI. In the coronal plane, note the preserved T2-hyperintense of the SV base (yellow arrow), which cleanly tapers into the ejaculatory duct (blue arrow), with immediately adjacent T2 hypointense tumor signal (dashed white arrows). Path: Right SVI. Teaching Point: Abutment on imaging may turn out to be invasion at path. |
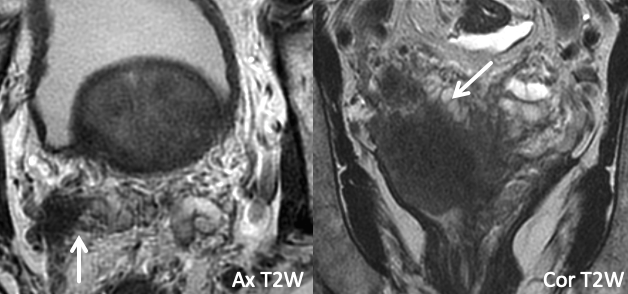
| Example 4 | Example 5 |
 |
 |
| Imaging Findings: Frank extraprostatic tumor secondarily invades the mid-portion of the right SV. | maging Findings: Primary lesion is in the left posteromedial/posterolateral basal peripheral zone. Two separate foci of restricted diffusion and intense focal enhancement are seen in the left and right SV, indicating discontinuous SVI. Path: Bilateral SVI. Teaching point: Focal SV implants separate from the primary lesion can uncommonly occur. Additionally, the findings were less conspicuous on T2W images, and multisequence assessment of the SV on T2W, DWI, and DCE may improve sensitivity for SVI. |
Clinical Significance of Extraprostatic Extension and Seminal Vesicle Invasion
- EPE (stage T3a) and SVI (stage T3b) worsen prognosis and increase risk of biochemical recurrence23
- Relevance to surgeons24
- EPE and SVI increase risk of a positive surgical margin (R1) – most common locations for positive margin also happen to be areas most prone to EPE (posterolateral base, apex)
- Can impact decision regarding nerve sparing, if EPE seen in region of neurovascular bundle
- Relevance to radiation oncologists25
- MRI is a useful tool for identifying EPE/SVI prior to definitive radiation therapy, given absence of a prostatectomy specimen
- EPE and SVI may triage patients respectively to “high risk” or ”very high risk” disease by NCCN classification, which may impact management via:
- Longer duration of androgen deprivation therapy
- Possible modification of the radiation plan (e.g. nodal irradiation, brachytherapy)
Clinically relevant anatomic landmarks should be scrutinized once a focal prostatic lesion is identified. Multiplanar assessment on T2W imaging is useful, as some relationships may not be evident in the axial plane.
| Key Local Staging Landmarks by Lesion Location | |
| Base | Seminal vesicles, bladder neck |
| Apex | External urethral sphincter |
| Posterolateral PZ | Neurovascular bundle |
| Anterior Gland | Anterior fibromuscular stroma |
| Any Location | Periprostatic tissues (EPE) |
External Urethral Sphincter18,24,26
- Anatomic and imaging principles
- External urethral sphincter (EUS) = striated muscle primarily encircling the membranous urethra, appearing as T2 hypointense periurethral signal just caudal to the prostatic apex
- Muscle fibers blend with prostatic apex to form an indistinct interface with deficient “capsule”, facilitating tumor spread18,26
- Tumor signal extending caudal to the apical prostatic margin along the urethra implies EUS invasion
- Broad tumor abutment of the caudal apical prostatic margin also increases risk of microscopic EUS involvement24
- Surgical Relevance
- EUS preservation at prostatectomy relates to continence, and frank EUS invasion may impact prostatectomy plans24
- Abutment of the apical prostatic margin/EUS should be scrutinized, even in the absence of frank EUS invasion.
| Normal External Urethral Sphincter |
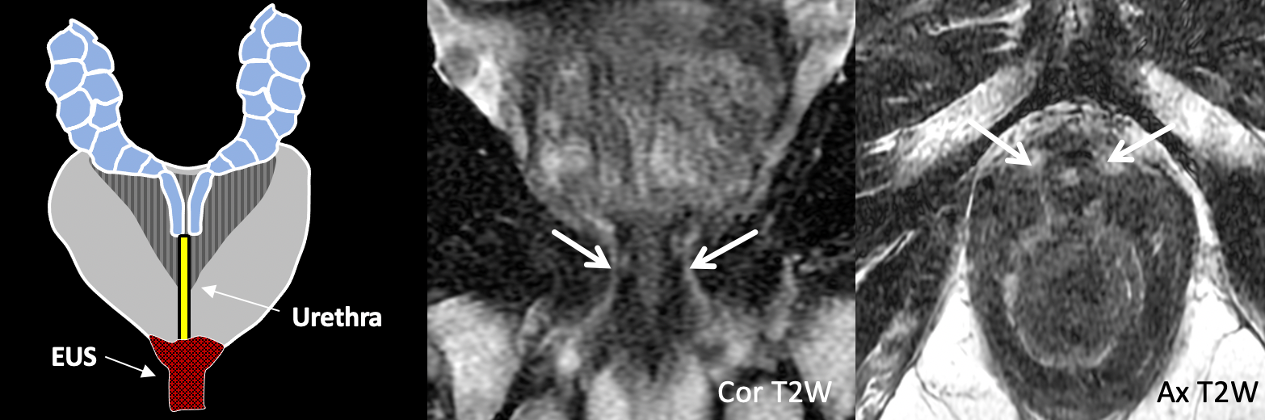 |
| T2-hypointense periurethral band of signal, just beyond the prostatic apex |
| Example 1 |
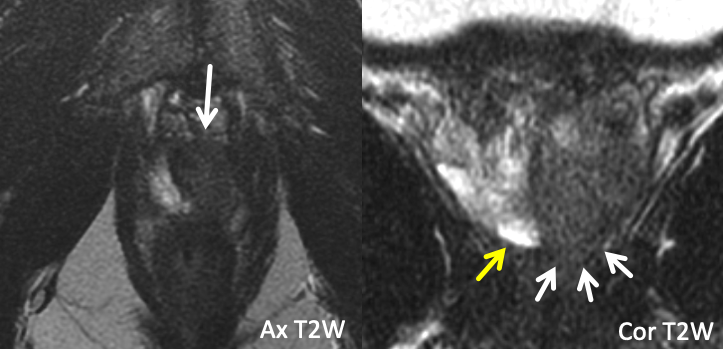 |
| Findings: T2-hypointense tumor signal (white arrows) extends beyond the apical prostatic margin (yellow arrow), seen best in the coronal plane Path: Positive distal surgical margin at the left apex |
Bladder Neck
- Anatomic and imaging principles
- Bladder neck = tapered portion of the bladder at its junction with the urethra and prostate, which contains the bulk of the internal urethral sphincter muscle
- Common to have prostatic tissue protrude into the bladder neck in the setting of BPH
- Tumor signal extending into the very T2 hypointense signal of the detrusor muscle implies bladder neck invasion; broad abutment should also be scrutinized, even in the absence of frank invasion24.
- Surgical relevance
- Some degree of bladder neck preservation during prostatectomy may relate to continence27
- Bladder neck invasion or abutment on imaging may impact the surgical plan
| Normal Bladder Neck |
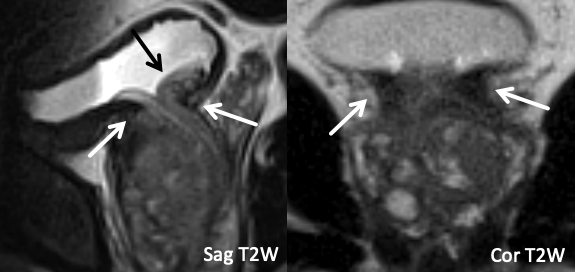 |
|
| Example 1 | Example 2 |
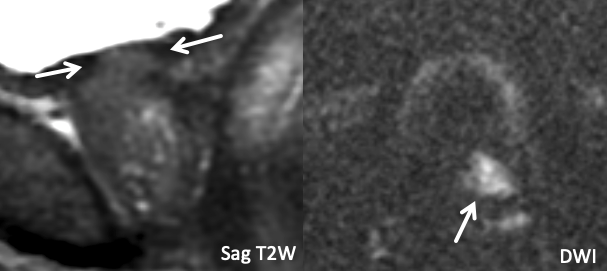 |
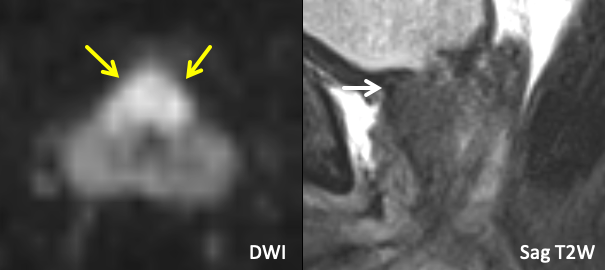 |
|
Findings: T2 iso-to-hypointense, diffusion restricting tumor signal replacing some of the normal T2 hypointense detrusor muscle in the bladder neck, consistent with frank bladder neck invasion. |
Findings: Large diffusion-restricting anterior base TZ lesion. Tumor signal abuts the bladder neck (white arrow) without frankly replacing the normal T2 hypointense detrusor signal, best seen in the sagittal plane. |
Neurovascular Bundles
- Anatomic and imaging principles
- Nerve fibers that contribute substantially to erectile function are organized most densely adjacent to the posterolateral prostatic capsule (though sometimes spread more fan-like across a larger area), and along with adjacent venous branches are collectively termed the neurovascular bundle (NVB)24,28
- NVBs are variably visualized on MRI29, but typically appear as a cluster of punctate/linear T2-hypointense foci at approximately 5 and 7 o’clock; the MRI-visible structures are likely predominantly small venous branches, though this allows one to infer the likely location of more densely concentrated periprostatic nerve fibers.
- Asymmetric irregular prominence of a NVB adjacent to an area of PZ tumor suggests NVB involvement, particularly if EPE is present
- Indirect signs of EPE (broad abutment, contour bulge, contour irregularity) adjacent to the expected area of the NVB (5 and 7 o’clock) are important to mention, particularly at the prostatic base, where there is a high rate of perineural spread along penetrating nerve fibers (“superior pedicle” of nerve fibers enters gland at this location)11,24
- Surgical Relevance
- Radical prostatectomy commonly involves bilateral “nerve-sparing”, where both the NVBs and adjacent fascia are preserved, relating to erectile function24
- Frank NVB involvement by tumor or direct/indirect signs of EPE near the NVB may impact decision regarding nerve-sparing24
| Normal Neurovascular Bundle | Example of NVB Involvement |
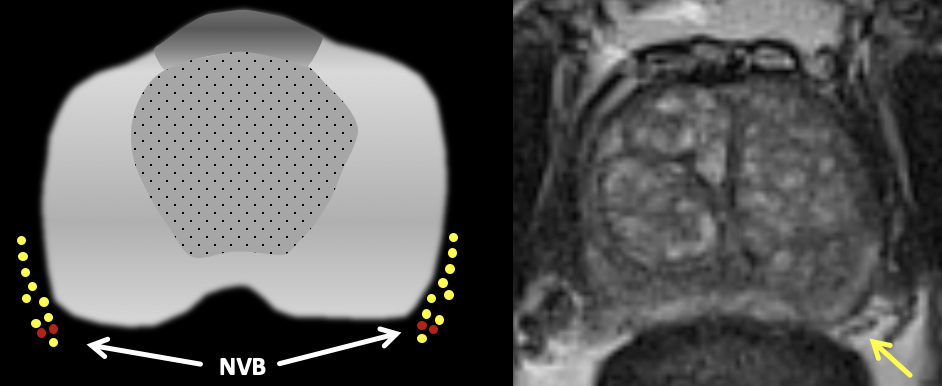 |
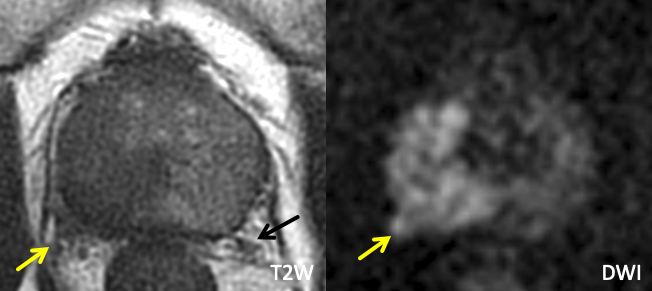 |
| Normal neurovascular bundle is visualized as a cluster of punctate/linear T2-hypointense foci (yellow arrow) |
Findings: Large right base PZ lesion with asymmetric T2 hypointense thickening along the right NVB (yellow arrow), with corresponding restricted diffusion. Note the normal contralateral NVB (black arrow). |
Anterior Fibromuscular Stroma
- Anatomic and imaging principles
- Anterior fibromuscular stroma (AFS) = band of non-glandular fibromuscular tissue blanketing the anterior surface of the prostate, contiguous with the muscle fibers of the EUS inferiorly (“EUS apron”) and detrusor superiorly (“detrusor apron”)18
- Variably visualized as a symmetric T2 hypointense band anterior to the TZ, with mild or no restricted diffusion and either no enhancement or low-level delayed enhancement on DCE30
- When inconspicuous, the location of the AFS is inferred as being along the anterior-most margin of the gland
- Anterior TZ lesions have a striking propensity for AFS involvement; therefore, tumor that appears centered in the AFS is most often related to an underlying anterior TZ lesion11
- AFS invasion is suggested when tumor directly extends into a well-visualized AFS, or when tumor abuts the anterior prostatic margin (even if AFS is not discretely visualized)
- Lesions involving the AFS and abutting and anterior gland have a higher risk of anterior EPE, due to relative anatomic weakness in this location19
- Surgical relevance
- AFS invasion and/or anterior prostate border abutment may modify the surgical plan with regard to sparing of anterior periprostatic supporting tissues31
| Normal AFS | Normal AFS |
 |
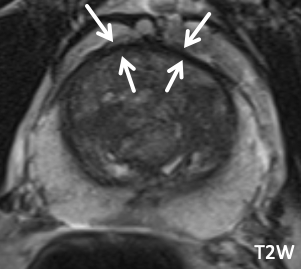 |
| Prominent but normal AFS with typical signal characteristics, including symmetric T2 hypointensity, lack of restricted diffusion, and lack of enhancement on DCE | Example of a normal AFS that is visible but less conspicuous |
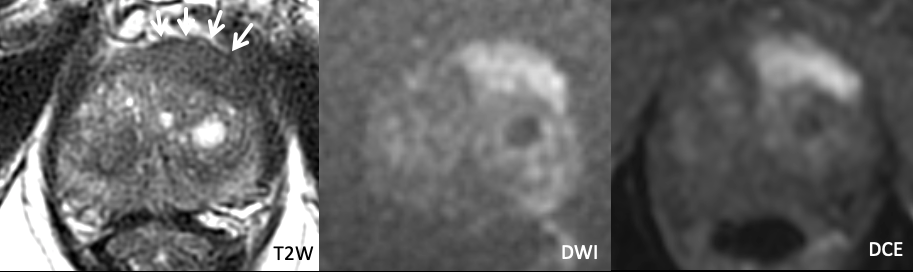
| Example 1 |
 |
|
Findings: Asymmetric T2 hypointense signal along the left anterior gland centered in the AFS with associated marked restricted diffusion and intense enhancement on DCE. PI-RADS 5. |
PSA Density
- Definition:
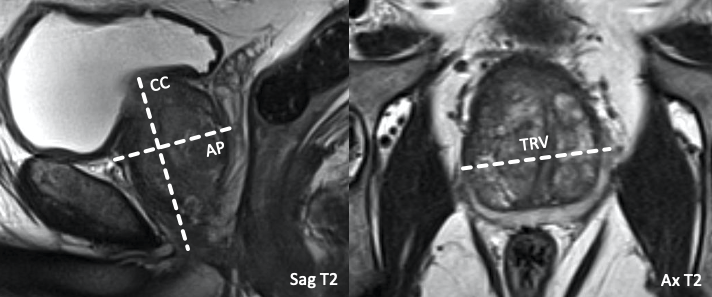
- Quotient of serum PSA level (on closest date available to MRI) divided by the prostate gland volume.
- Volume (ml) may be estimated using ellipsoid volume calculation (CC x AP x TRV x 0.52)
- Concept
- Corrects PSA for size of gland, given that a larger gland would be expected to have a higher PSA
- PSA density > 0.1-0.15 ng/ml2 increases the pre-test probability of clinically significant prostate cancer, though is not entirely sensitive or specific32,33.
- Interface with MRI
- Abnormally elevated PSA density should heighten suspicion for clinically significant prostate cancer
- May impact urologist’s decision of whether or not to perform biopsy32,33
- PI-RADS Manual, Version 2.1, 2019.
- McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897-906. doi:10.1097/00000478-198812000-00001
- Khan FU, Ihsan AU, Khan HU, et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017;94:1064-1076. doi:10.1016/j.biopha.2017.08.016
- Rosenkrantz AB, Taneja SS. Radiologist, be aware: Ten pitfalls that confound the interpretation of multiparametric prostate MRI. Am J Roentgenol. 2014;202(1):109-120. doi:10.2214/AJR.13.10699
- Thomas S, Oto A. Multiparametric MR imaging of the Prostate: Pitfalls in Interpretation. Radiol Clin North Am. 2018;56(2):277-287. doi:10.1016/j.rcl.2017.10.009
- Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A. Benign conditions that mimic prostate carcinoma: MR imaging features with histopathologic correlation. Radiographics. 2016;36(1):162-175. doi:10.1148/rg.2016150030
- Gottlieb J, Princenthal R, Cohen MI. Multi-parametric MRI findings of granulomatous prostatitis developing after intravesical bacillus calmette–guérin therapy. Abdom Radiol. 2017;42(7):1963-196 doi:10.1007/s00261-017-1081-z
- Kawada H, Kanematsu M, Goshima S, et al. Multiphase contrast-enhanced magnetic resonance imaging features of bacillus calmette-guérin-induced granulomatous prostatitis in five patients. Korean J Radiol. 2015;16(2):342-34 doi:10.3348/kjr.2015.16.2.342
- Panebianco V, Barchetti F, Barentsz J, et al. Pitfalls in Interpreting mp-MRI of the Prostate: A Pictorial Review with Pathologic Correlation. Insights Imaging. 2015;6(6):611-630. doi:10.1007/s13244-015-0426-9
- Lee JJ, Thomas IC, Nolley R, Ferrari M, Brooks JD, Leppert JT. Biologic differences between peripheral and transition zone prostate cancer. Prostate. 2015;75(2):183-190. doi:1002/pros.22903
- McNeal JE, Haillot O. Patterns of spread of adenocarcinoma in the prostate as related to cancer volume. Prostate. 2001;49(1):48-57. doi:10.1002/pros.1117
- Lewis S, Besa C, Rosen A, et al. Multiparametric magnetic resonance imaging for transition zone prostate cancer: essential findings, limitations, and future directions. Abdom Radiol. 2017;42(11):2732-2744. doi:10.1007/s00261-017-1184-6
- Chesnais AL, Niaf E, Bratan F, et al. Differentiation of transitional zone prostate cancer from benign hyperplasia nodules: Evaluation of discriminant criteria at multiparametric MRI. Clin Radiol. 2013;68(6). doi:10.1016/j.crad.2001.018
- Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng FM, Taneja SS. Transition zone prostate cancer: Revisiting the role of multiparametric MRI at 3 T. In: American Journal of Roentgenology. Vol 204. American Roentgen Ray Society; 2015:W266-W272. doi:10.2214/AJR.12955
- Penzkofer T, Tuncali K, Fedorov A, et al. Transperineal in-bore 3-T MR imaging-guided prostate biopsy: A prospective clinical observational study. Radiology. 2015;274(1):170-180. doi:10.1148/radiol.14140221
- Yacoub JH, Oto A. MR Imaging of Prostate Zonal Anatomy. Radiol Clin North Am. 2018;56(2):197-209. doi:10.1016/j.rcl.2017.10.003
- Duvnjak P, Schulman AA, Holtz JN, Huang J, Polascik TJ, Gupta RT. Multiparametric Prostate MR Imaging: Impact on Clinical Staging and Decision Making. Urol Clin North Am. 2018;45(3):455-466. doi:10.1016/J.UCL.2018.03.010
- Vilanova JC (Joan C., Catalá V, Algaba F, Laucirica O. Atlas of Multiparametric Prostate MRI : With PI-RADS Approach and Anatomic-MRI-Pathological Correlation.
- Kang YJ, Abalajon MJ, Jang WS, et al. Association of anterior and lateral extraprostatic extensions with base-positive resection margins in prostate cancer. PLoS One. 2016;11(7). doi:10.1371/journal.pone.0158922
- de Rooij M, Hamoen EHJ, Witjes JA, Barentsz JO, Rovers MM. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol. 2016;70(2):233-245. doi:10.1016/j.eururo.2015.07.029
- Mehralivand S, Shih JH, Harmon S, et al. A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric mri. J Urol. 2019;202(3):440-441. doi:10.1148/radiol.2018181278
- Valentin B, Schimmöller L, Ullrich T, et al. Magnetic resonance imaging improves the prediction of tumor staging in localized prostate cancer. Abdom Radiol. 2021. doi:10.1007/s00261-020-02913-9
- Ball MW, Partin AW, Epstein JI. Extent of extraprostatic extension independently influences biochemical recurrence-free survival: Evidence for further PT3 subclassification. Urology. 2015;85(1):161-164. doi:10.1016/j.urology.2014.08.025
- McEvoy SH, Raeside MC, Chaim J, Ehdaie B, Akin O. Preoperative prostate MRI: A road map for surgery. Am J Roentgenol. 2018;211(2):383-391. doi:10.2214/AJR.17.18757
- NCCN Guidelines. Prostate Cancer. Version 1.2021.
- Koraitim MM. The Male Urethral Sphincter Complex Revisited: An Anatomical Concept and its Physiological Correlate. J Urol. 2008;179(5):1683-1689. doi:10.1016/j.juro.2008.01.010
- Ma X, Tang K, Yang C, et al. Bladder neck preservation improves time to continence after radical prostatectomy: A systematic review and meta-analysis. Oncotarget. 2016;7(41):67463-67475. doi:10.18632/oncotarget.11997
- Park YH, Jeong CW, Lee SE. A comprehensive review of neuroanatomy of the prostate. Prostate Int. 2013;1(4):1-7. doi:10.12954/pi.13020
- Sklinda K, Frączek M, Mruk B, Walecki J. Normal 3T MR Anatomy of the Prostate Gland and Surrounding Structures. Adv Med. 2019;2019:1-9. doi:10.1155/2019/3040859
- Ward E, Baad M, Peng Y, et al. Multi-parametric MR imaging of the anterior fibromuscular stroma and its differentiation from prostate cancer. Abdom Radiol. 2017;42(3):926-934. doi:10.1007/s00261-016-0951-0
- Wagaskar VG, Mittal A, Sobotka S, et al. Hood Technique for Robotic Radical Prostatectomy—Preserving Periurethral Anatomical Structures in the Space of Retzius and Sparing the Pouch of Douglas, Enabling Early Return of Continence Without Compromising Surgical Margin Rates. Eur Urol. 2020. doi:10.1016/j.eururo.2020.09.044
- Stevens E, Truong M, Bullen JA, Ward RD, Purysko AS, Klein EA. Clinical utility of PSAD combined with PI-RADS category for the detection of clinically significant prostate cancer. Urol Oncol Semin Orig Investig. 2020;38(11):846.e9-846.e16. doi:10.1016/j.urolonc.2020.05.024
- Padhani AR, Barentsz J, Villeirs G, et al. PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology. June 2019:182946. doi:10.1148/radiol.2019182946