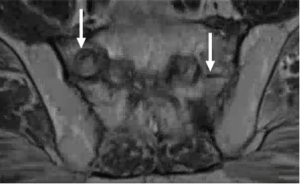
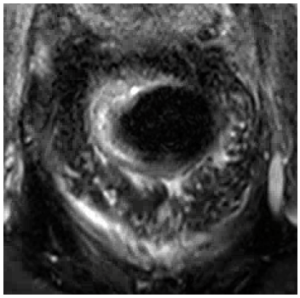
Post-treatment MRI Evaluation of Rectal Cancer
Authors: Renata Rocha de Almeida, Shanna Matalon MD, Daniel Souza, Leslie Lee, Stuart Silverman
Date: December 2021
Indication for neoadjuvant chemoradiation based on current NCCN guidelines:
Stages II & III rectal cancer:
- T3 (invades perirectal tissues) or T4 (invades adjacent organs), with any N disease,
- N1 or N2 disease (at least one regional node/deposit), with any T disease,
- Locally unresectable or medically inoperable tumors
- Some low rectal tumors to allow for sphincter sparing resection
Current neoadjuvant chemotherapy options: 5-FU (infusional fluorouracil or oral capecitabine), oxaliplatin, +/- leucovorin, +/- irinotecan.
Neoadjuvant radiation:
- Long course: ~50 Gy in 2528 fractions daily over 5-6 weeks + radiosensitizing chemotherapy (5-FU or capecitabine)
- Short course: 25 Gy in 5 fractions over 1 week (without chemotherapy)
While highest radiation dose is aimed at the rectum, other organs also receive high doses of radiation, such as the bladder, sacrum, pre-sacral fat, and prostate. Lower radiation fields may be used to encompass inguinal lymphadenopathy in the setting of low rectal tumors.
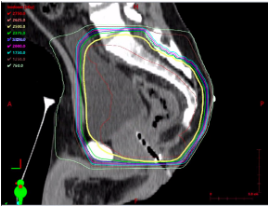
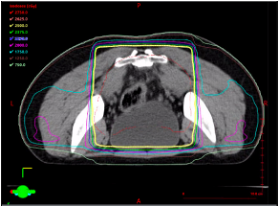
“Total neoadjuvant therapy” (TNT): All chemotherapy and radiation therapy given prior to surgery, with various permeations available. May be used for any of the following indications: any T4, any N2, tumors with threatened resection margin, and some low rectal tumors to allow for sphincter sparing resections.
| Sequence | Area of interest |
|
Coronal T2 |
Screen for metastases |
|
Sagittal T2 |
Prescribe oblique planes |
|
Short axis oblique T2* |
Assess primary tumor and lymph nodes |
|
Long axis oblique T2/coronal T2 |
Relationship of primary tumor to sphincter complex (low cancers) |
|
DWI & ADC* |
Assess for persistent diffusion restriction of residual tumor |
|
Post contrast** |
Assess for persistent enhancement of residual tumor and heterogeneity of lymph nodes |
*Key sequence to assess residual tumor
Prescribing oblique planes for angled T2-weighted images:
- Provides optimal anatomic information
- Reduces volume averaging
| Tumor may be difficult to identify after treatment | Look at the pre-treatment MRI to identify tumor location | Draw oblique lines in the form of a “fishbone” based on the long and short axis of tumor |
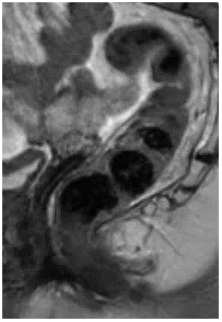 |
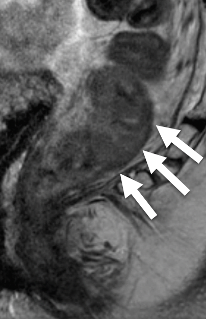 |
 |
MRI Features of Treatment Response
Treatment response features on T2:
- Decrease or resolution of T2-intermediate signal of viable tumor
- Development of T2-hypointense fibrosis within the rectal wall or adjacent mesorectal fat (desmoplastic reaction)
- Acellular mucin production: new or increased T2-hyperintense signal, similar to water
1. Fibrosis
| Initial Staging MRI – T2WI | After Neoadjuvant Treatment MRI – T2WI |
Comment | |
|---|---|---|---|
| Small / focal | 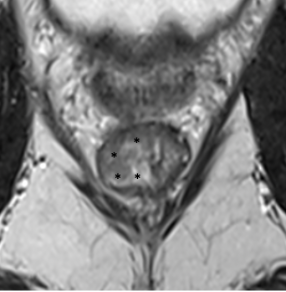 |
 |
Well-defined thin T2-hypointense signal (arrow) Fibrosis confined to the rectal wall Complete response |
|
Semicircular/full-thickness |
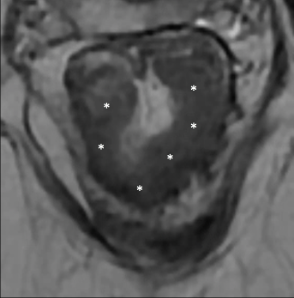 |
 |
Thick T2-hypointense signal (*) Fibrosis involves the full thickness of rectal wall (*) +/- Desmoplastic reaction (Arrow: T2-hypointense lines in mesorectal fat) Usually complete response |
| Spiculated | 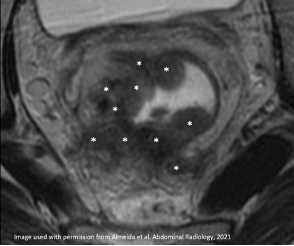 Irregular spiculated tumor (*) |
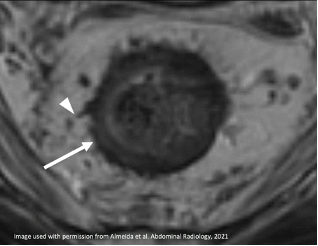 |
Spiculated T2-hypointense signal (arrow) Desmoplastic reaction (arrowhead) More frequent residual tumor |
2. Mucinous degeneration in non-mucinous adenocarcinoma
| Initial MRI – T2WI | During Neoadjuvant Treatment – T2WI | After Neoadjuvant Treatment – T2WI |
|---|---|---|
 |
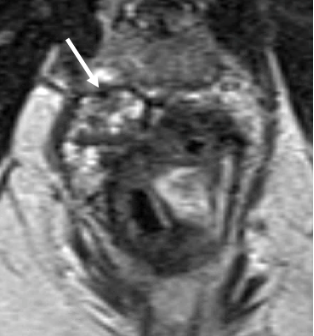 |
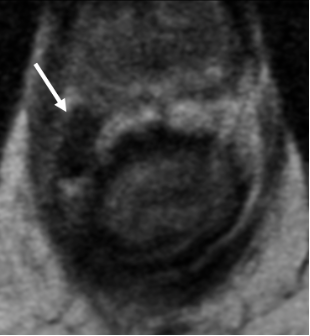 |
| Nodular T2-intermediate tumor signal (arrow) | Replaced by T2-hyperintense signal of mucin (arrow) | May be replaced by T2-hypointense scar (arrow) |
3. Increased mucin in mucinous/signet ring cell cancers
| Subtype | Initial MRI | After Neoadjuvant Treatment |
|---|---|---|
| Mucinous adenocarcinoma |
T2-hyperintense signal reflects acellular /nonviable tumor |
Increased T2-hyperintense signal |
| Signet-ring cell adenocarcinoma | 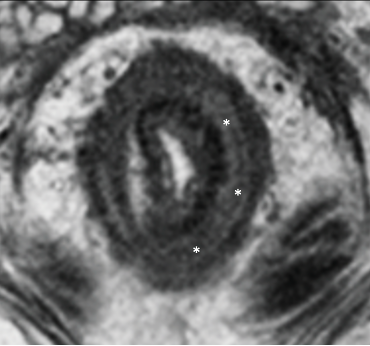 |
 |
Treatment response features on DWI & ADC:
- Resolved or decreased extent of restricted diffusion in tumor bed
- Increased ADC signal and decreased DWI signal in tumor bed
- Signal on DWI is similar to ADC (high-DWI & high-ADC OR low-ADC & low-DWI)
Non-mucinous Adenocarcinoma
| Sequence | Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|---|
| T2 | 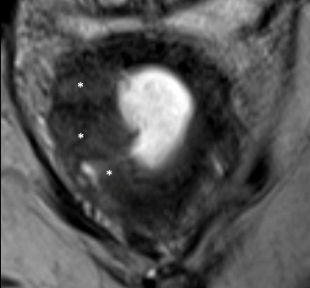 T2-intermediate tumor (*) |
 |
Increased signal in tumor bed (*) due to acellular mucin production |
| DWI | 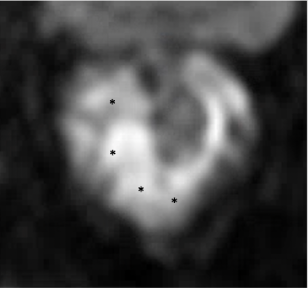 DWI hyperintense tumor (*) |
 |
Decreased signal in tumor bed (*) due to loss of cellularity |
| ADC |  ADC hypointense tumor (*) |
 |
Increased signal in tumor bed (*) due to loss of cellularity |
MRI Features of Residual Tumor
Residual tumor features on T2 and T1FSC+:
- Persistent nodular-shaped T2-intermediate signal in tumor bed (in non-mucinous tumors)
- Persistent T2-intermediate signal in submucosa (signet-ring cell subtype)
- Early enhancement tumor bed ≥ than initial MRI
- Unchanged or minimally decreased cellular mucin (in mucinous tumors)
1. Residual disease in a non-mucinous adenocarcinoma
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
 T2-intermediate tumor (arrow) |
 |
Persistent nodular-shaped T2-intermediate signal in tumor bed (arrow) |
 Hyperenhancing tumor (*) |
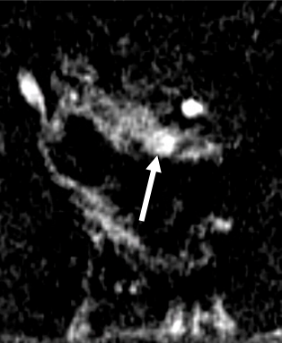 |
Early enhancement tumor bed (arrow) > than initial MRI |
2. Residual disease in a mucinous asdenocarcinoma
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
 T2-hyperintense tumor (arrows) Internal intermediate signal (*) |
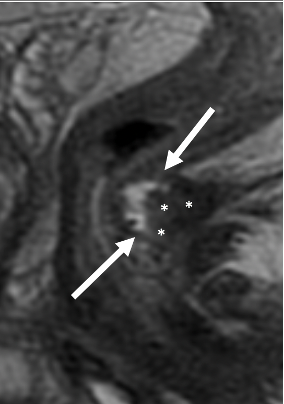 |
Decreased T2-hyperintense mucinous tumor (arrows) Persistent T2-intermediate signal (*) of cellular mucin |
3. Residual disease in a signet ring cell adenocarcinoma
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
 T2-intermediate tumor in submucosa (*) |
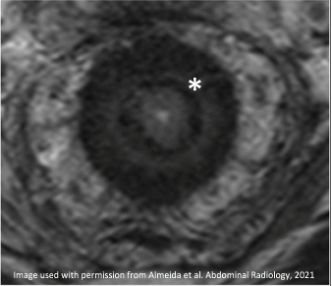 |
Improved but persistent T2-intermediate signal of cellular mucin in mucinous tumor |
Reassessment of Circumferential Resection Margin (CRM)
Reassessment of mesorectal fascia (MRF) involvement
- Resolved MRF involvement: Development of clear fat plane (≥ 2mm) ± desmoplastic reaction between tumor and MRF
- Persistent MRF involvement: Persistent T2-intermediate or T2-hyperintense signal infiltration of MRF
- Indeterminate MRF involvement: Diffuse fibrotic (T2-hypointense) infiltration of MRF
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
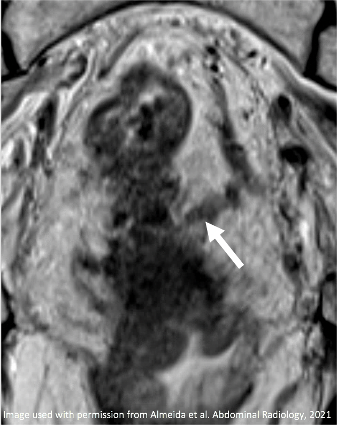
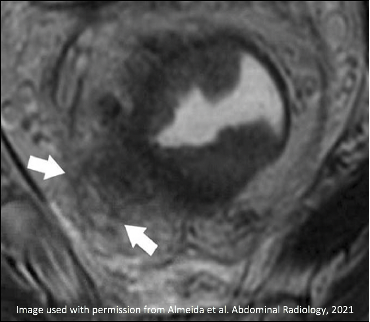 T2-intermediate tumor involves MRF (arrows) |
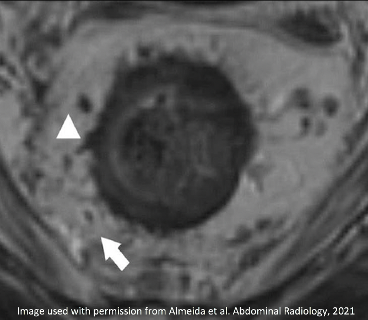 |
New fat plane (arrow) with desmoplastic reaction (arrowhead) between tumor bed and MRF Resolved MRF Involvement |
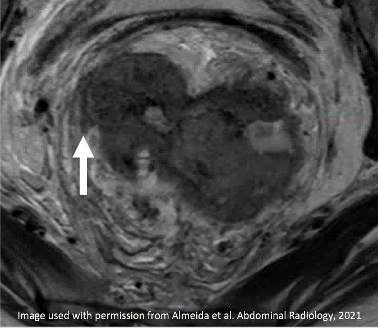 T2-intermediate tumor involves MRF (arrow) |
 |
Persistent T2-intermediate signal of tumor along MRF (arrow) Persistent MRF Involvement |
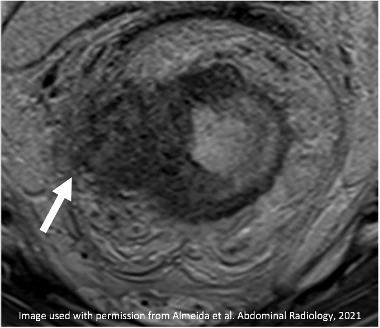
 T2-intermediate tumor involves MRF (arrow) |
 |
Thick T2-hypointense signal of fibrosis along MRF (arrow) Indeterminate MRF Involvement |
Reassessment of sphincter complex in low rectal cancers
- Allow sphincter-sparing surgery: Tumor involves up to the intersphincteric fat but does not involve external sphincter; > 2 cm from anal verge
- Does not allow sphincter-sparing surgery: Tumor invades external sphincter; < 1-2 cm from anal verge
Reassessment of extramural vascular invasion
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
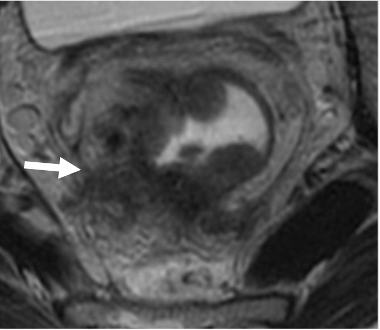
 T2-intermediate signal along vessels (arrow) |
 |
Resolved T2-intermediate signal along mesorectal vessels Replacement by linear T2-hypointense fibrosis (arrow) Resolved extramural vascular invasion (EMVI) |
 T2-intermediate signal along vessels (arrow) |
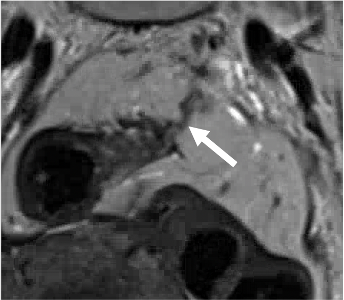 |
Improved but persistent nodular T2-intermediate signal along mesorectal vessels (arrow) Persistent extramural vascular invasion (EMVI) |
Locoregional nodes (N1/2): Mesorectal, superior rectal, obturator (posterior to external iliac vessels), and internal iliac stations (and inguinal nodes, sometimes considered locoregional if a low rectal cancer involving anal canal).
- “Lateral lymph nodes”: Refers to obturator and internal iliac stations, which are not removed with standard surgical resection; it is important to report these separately as at some institutions, a lateral lymph node dissection will also be performed.
Non–locoregional nodes (M1): external iliac (anterior or lateral to external iliac vessels), common iliac, and paraaortic, and inguinal stations (if mid or high rectal cancer)
MRI Features of Nodal Treatment Response
- Disappearance or decreased size to < 5mm short axis
- Replacement by T2-hypointense fibrosis
- New/Increased T2-hyperintense signal of acellular mucin
| Initial MRI | After Neoadjuvant Treatment | Comment |
|---|---|---|
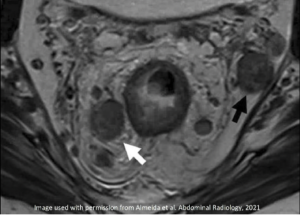 Enlarged and heterogeneous mesorectal (white arrow) and internal iliac nodes (black arrow) |
 |
Complete disappearance of mesorectal and internal iliac nodes Replacement by T2-hypointense fibrosis (arrow) |
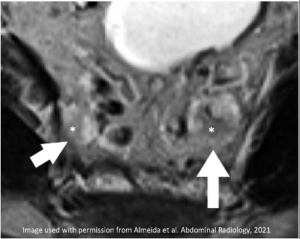 Enlarged & T2 hyperintense superior rectal nodes (arrows) with internal T2-intermediate signal (*) |
 |
New/Increased T2-hyperintense signal of acellular mucin (arrow) Decreased size (arrowhead) |
MRI Features of Residual Tumor in Nodes
- Size ≥ 5 mm in short axis (**only feature considered by SAR/ESGAR panels)
- Persistent T2-intermediate signal, irregular contour, heterogeneous signal
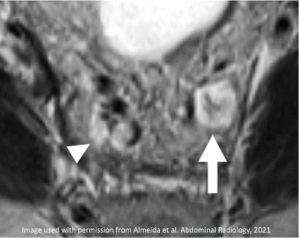
| Initial MRI | After Neoadjuvant Treatment |
|---|---|
 Enlarged lymph nodes with T2-intermediate signal and irregular contour in the obturator (white arrow) and internal iliac (black arrow) stations. O=Obturator muscle |
 Most nodes have decreased size but there is a persistent node with ≥ 5 mm in short axis and irregular contour (white arrow). O=Obturator muscle |
Pseudotumor
- Pseudotumor is a mucosal edema after radiation involving the rectal wall not previously involved by tumor, appearing as T2-hyperintense signal
| Initial MRI – T2WI | After Neoadjuvant Treatment – T2WI |
|---|---|
 T2-intermediate tumor invading the mesorectal fat at 12 o’clock (arrow) with heterogeneous tumor deposits (arrowheads) |
 T2-hypointense fibrosis in rectal wall at 12 o’clock (arrow). New adjacent T2-hyperintense masslike thickening posteriorly (*) (Pseudotumor) |
Persistent restricted diffusion versus T2-shine-through OR T2-dark-through
- T2-shine-through and T2-dark-through are seen in tumor response
- T2-shine-through (hyperintense signal on DWI and ADC) happens due to decreased cellularity at the tumor bed
- T2-dark-through (hypointense signal on DWI and ADC) happens due to increased cellularity of dense fibrosis replacing the tumor bed
- Tip: Equal (low-low or high-high) signal in DWI and ADC means response rather than viable tumor
T2-shine-through in a patient with a complete response
| Sequence | Initial MRI | After Neoadjuvant Treatment |
|---|---|---|
| T2WI |  Circumferential T2-intermediate tumor (*) |
 Persistent T2-intermediate signal in tumor bed (*) raises question of residual tumor |
|
DWI |
 DWI hyperintense tumor (*) |
 Persistent DWI hyperintense signal (*) in tumor bed also raises question of residual tumor |
| ADC |  ADC hypointense tumor (*) |
 New ADC hyperintense signal in tumor bed due to T2-shine-through and treatment response |
T2-dark-through in a patient with complete response
| Sequence | Initial MRI | After Neoadjuvant Treatment |
|---|---|---|
| T2WI |  T2-intermediate tumor (*) |
 New T2-hypointense signal in tumor bed (*) due to fibrosis |
|
ADC |
 ADC hypointense tumor (*) |
 Persistent ADC hypointense signal in tumor bed (*) raises question of residual tumor |
|
DWI |
 DWI hyperintense tumor (*) |
 New DWI hypointense signal in tumor bed (*) due to T2-dark-through |
|
Fistulas: |
Proctitis, Enteritis, Colitis: |
|
Insufficiency Fracture: |
Pre-sacral and mesorectal edema: |
Other (not shown): Avascular necrosis of femoral heads, Cystitis
- Almeida RR, Souza D, Matalon SA, Hornick JL, Lee LK, Silverman SG. Rectal MRI after neoadjuvant chemoradiation therapy: a pictorial guide to interpretation. Abdom Radiol (NY). 2021 Jul;46(7):3044-3057.
- Park S, Joon Seok Lim M, Lee J, et al. Rectal Mucinous adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy—A Hypothesis- generating Study. Radiology. 2017;285(285):124–133.
- Lambregts DMJ, Pizzi AD, Lahaye MJ, et al. A pattern-based approach combining tumor morphology on MRI with distinct signal patterns on diffusion-weighted imaging to assess response of rectal tumors after chemoradiotherapy. Dis Colon Rectum.
- 2018;61:328–337.
- Lambregts DMJ, Boellaard TN, Beets-Tan RGH. Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: a pictorial review. Insights Imaging. 2019;10.
- Kalis KR, Enzerra MD, Paspulati RM. MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy.
- Radiographics. 2019;39:538–556.
- Amin M, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. American Joint Committee on Cancer. New York: Springer; 2017. p. 252–254.
- Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol. 2018;43:2893–2902.
- Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–1475.