COVID-19
Authors: Sona Chikarmane, MD, Lisa Frost, MD, Cathy Giess, MD, Ramin Khorasani, MD, MPH
Date: March 10, 2021
BWH/DFCI Breast Imaging Management of Axillary Adenopathy After Recent (<6 weeks) COVID-19 Vaccination
| Patient Population | Principles | Imaging Finding |
| 1.Asymptomatic screening mammography |
Encourage already scheduled screening (no delay) of all patients regardless of vaccination status. At time of imaging, document vaccination status (1st or 2nd dose, location (leg or arm), side, date). |
If negative breast imaging with adenopathy ipsilateral to the COVID-19 vaccine:
Vaccination status, imaging findings, and recommendations will be documented in imaging report, using certainty scale where appropriate *DFCI breast oncologists request yellow ANCR for these BIRADS 2 patients. |
| 2. Asymptomatic screening breast MRI |
Encourage already scheduled screening (no delay) regardless of vaccination status. At time of imaging, documentation of vaccination status (1st or 2nd dose, location (leg or arm), side, date). |
If negative breast imaging with unilateral adenopathy ipsilateral to arm which received the COVID-19 vaccination, give BI-RADS 3 (probably benign) assessment with follow-up ultrasound 6 weeks after last dose of vaccine.** (use blue ANCR) https://www.ajronline.org/doi/10.2214/AJR.21.25604?mobileUi=0 |
| 3. Symptomatic (in breast and/or axilla) |
Recommend diagnostic imaging of all patients with breast signs/symptoms regardless of vaccination status. In the setting of documented recent (<6w) COVID-19 vaccination and post vaccine palpable ipsilateral axillary adenopathy, in absence of breast signs/symptoms, clinical follow-up of axilla is recommended. If clinical concerns persist >6w after final vaccine dose, axillary US is recommended, with mammography if patient is due, or at discretion of radiologist. At time of imaging, documentation of vaccination status (1st or 2nd dose, location (leg or arm), side, date). |
Palpable isolated unilateral adenopathy >6w after vaccination is managed with standard imaging protocol of unilateral adenopathy (US +/- mammography). Unilateral adenopathy on side of vaccination, identified incidentally during diagnostic imaging work-up for breast sign/symptom:
|
| 4. Current breast cancer (pre/peri treatment) | Encourage recommended imaging regardless of vaccination status. Encourage contralateral arm injection. |
Unilateral adenopathy on side of (arm) vaccine and side of breast cancer: biopsy versus imaging/clinical follow-up at discretion of surgeon and/or medical or radiation oncologist. Unilateral adenopathy on side of vaccine and contralateral to breast cancer: will be managed like asymptomatic screening breast MRI patients with follow-up axillary ultrasound in 6-12 weeks. |
*BI-RADS 2 Assessment Workflow:
- Use “COVID scr TOMO BIRADS 2” template
- If DFCI referring provider, send yellow ANCR
- Otherwise, no ANCR or alert (use judgment for patients with history of breast cancer)
**BI-RADS 3 Assessment Workflow with blue ANCR:
- Use “COVID scr TOMO B3 us 6 weeks” template. Report includes the statement “Likely COVID-vaccine related adenopathy, 6-week ultrasound after second dose recommended.” For Recommendation, select “callback imaging”. For Recall Interval, select “at this time”. For type of imaging select the COVID option, which stipulates US of axilla but also states US should not be done until 6 weeks after 2nd dose.
- Send blue ANCR, and in the comment box state that US of axilla should not be scheduled until 6 weeks after 2nd dose.
- Insert certainty scale at bottom of report (place cursor, type “cs”, hit return and macro with link will populate into report)
***BI-RADS 0 Assessment Workflow with Pink Alert
- Follow the usual pink alert workflow for immediate callbacks.
From: COVID-Vaccine related lymphadenopathy Radiology working group
To: Brigham Health/DFCI Radiology Faculty, Fellows, Residents
RE: COVID-Vaccine related lymphadenopathy in non-breast imaging examinations
Date: March 15, 2021
A Brigham Health/DFCI Radiology working group was assembled among various radiology subspecialty divisions (members include Atul Shinagare, MD, Sona Chikarmane, MD, Heather Jacene, MD, Dan Glazer, MD, Mark Hammer, MD, Pam Dipiro, MD, Jeff Guenette, MD, Patrick Curley, Neena Kapoor, MD, Ramin Khorasani, MD, MPH) to review our current understanding of COVID vaccine-related lymphadenopathy and to make recommendations for addressing related imaging findings that may at times be challenging to distinguish from malignant etiologies for lymphadenopathy. With the accelerating pace of COVID vaccinations, an awareness of this entity and its imaging features will be helpful to reduce patients’ unwarranted fear and reduce potentially unnecessary additional diagnostic and interventional procedures.
Case Examples:
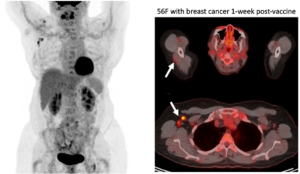
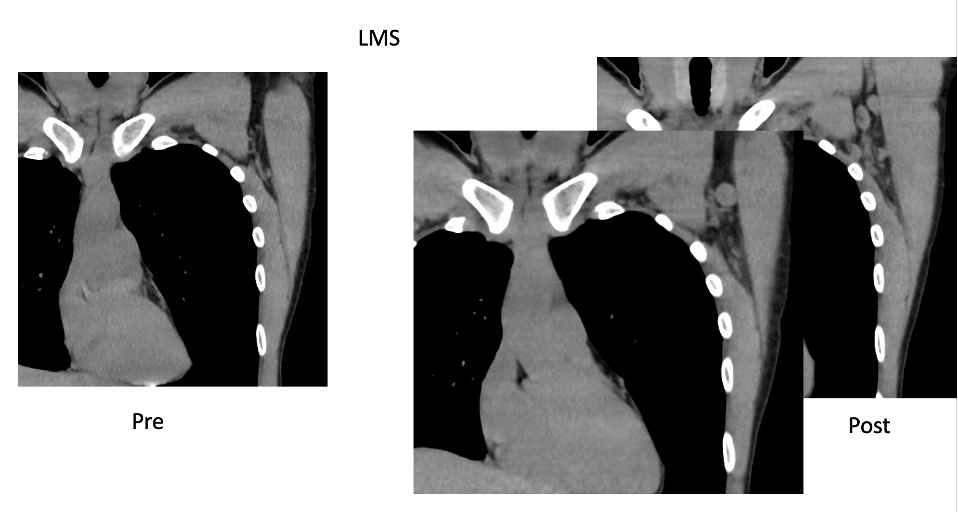
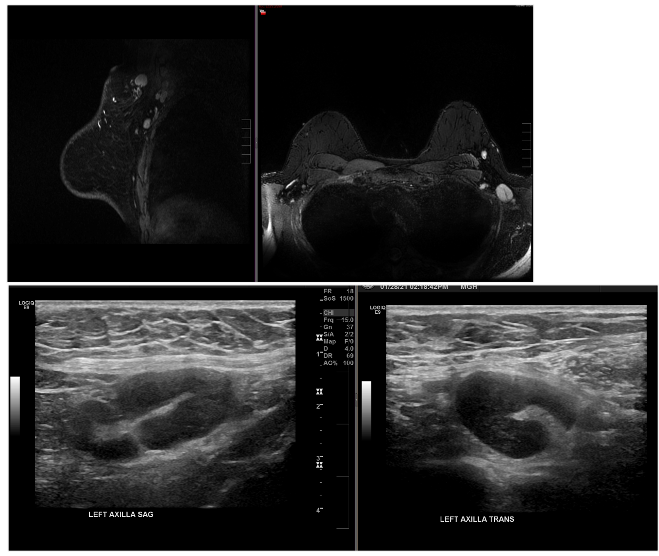
Case Courtesy Stephanie Chung, Sona Chikarmane, Neha Modi
BH-DFCI Workgroup Recommendations for Non-Breast Imaging Patients
Recommendations for Interpretation and Follow-up of asymterically enlarged lymph nodes
- Lymphadenopathy should be interpreted in the context of the site and time interval since vaccination and overall metastatic risk in patients with known primary malignancy (e.g., cancer type, location, stage, etc.) and in the clinical judgment of the interpreting radiologist.
- Patient’s COVID-19 immunization history is available in Epic (for all MGB vaccinations in addition to information from Masschusetts Vaccine registry which is expected to be updated in Epic within 24 hours, though we have seen several instances of missing information for the latter) by clicking the immunization icon in Epic (circled red below) which appears to the right of patient’s initials or picture.
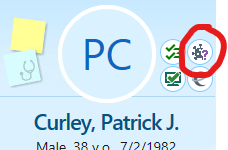
You can then review the immunization history-including COVID-19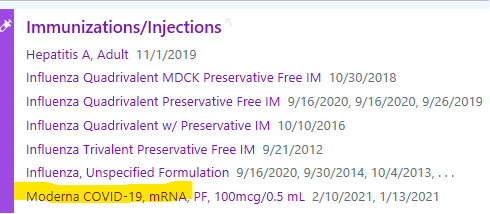
- In patients without a known primary malignancy, or with primary cancers with low risk of axillary or supraclavicular nodal metastases who have recent (within 6 week) vaccination in the ipsilateral arm:
- A. In the Impression use relevant certainty phrase to state that lymphadenopathy is likely/most likely due to COVID vaccine related lymphadenopathy.
- B. Insert Certainty Scale at the bottom of the Impression so patients and referring providers can follow the embedded link to better understand the meaning of likely/most likely.
- No need to make a follow up imaging recommendation.
- In patients with potentially high risk of metastatic lymphadenopathy (e.g., breast, head and neck, upper extremity/trunk melanoma or lymphoma) Follow-up is needed to ensure resolution of findings which may include physical exam for palpable nodes, ultrasound (or already scheduled routine follow-up imaging with patients on protocols) at least 6 weeks after the last vaccine done(second for Pfizer, Moderna, and the initial dose of Johnson and Johnson). The referring team may request pathology confirmation if immediate decision is necessary for patient management
- A. In the Impression Use relevant certaintly phrase (e.g., may represent or likely) to convey your subjective assessement for risk of COVID vaccine related lymphadenopathy vs malignancy.
- B. Based on your assessement in A, above, the referring provider, often an oncologist, will determine the most relevant follow up strategy in the context of shared decision making with the patient. If in your clinical judgment you feel follow up imaging is necessary, state follow up imaging recommendation and timeframe (use Blue ANCR to help ensure timely completion of follow up) AND/OR state tissue would be needed to make definitive diagnosis if you believe tissue sampling is prudent.
- C. Insert certainty scale at the end of report.
- Multi-disciplinary discussion may also be helpful in uncertain cases.