Tumor Response Assessment
Authors: Katherine Krajewski, Atul Shinagare, Richard Thomas
Date: January 11, 2021
- Radiologists are commonly asked to assess the effect of anti-cancer agents on tumor burden. This is achieved by the measurement of tumor lesions on baseline and follow-up imaging studies and overall response assessment at each timepoint according to widely accepted response criteria.
- In order to accurately characterize response to treatment with the appropriate response criteria, it is necessary to know
- The malignancy
- Type of therapy administered
It is also helpful to know
- When the therapy was initiated
- A sense of the ongoing clinical picture, which may be obtained from ordering information and/or oncology provider notes.
- Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) is the most commonly employed imaging response assessment. This can be applied to any malignancy treated with any agent. Response assessment is rendered based upon changes in target and non-target lesions.
- Response assessment is based on the change of the sum diameter of target lesions over time (long axes of target masses + short axes of target nodes). Timepoint sum diameters are compared to the baseline sum diameter or the nadir sum diameter (whichever is smaller).
- Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228-47.
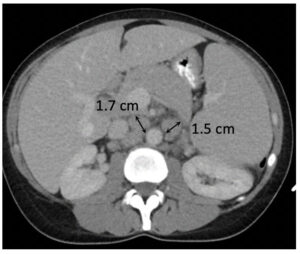
Measurable Target Lesions
|
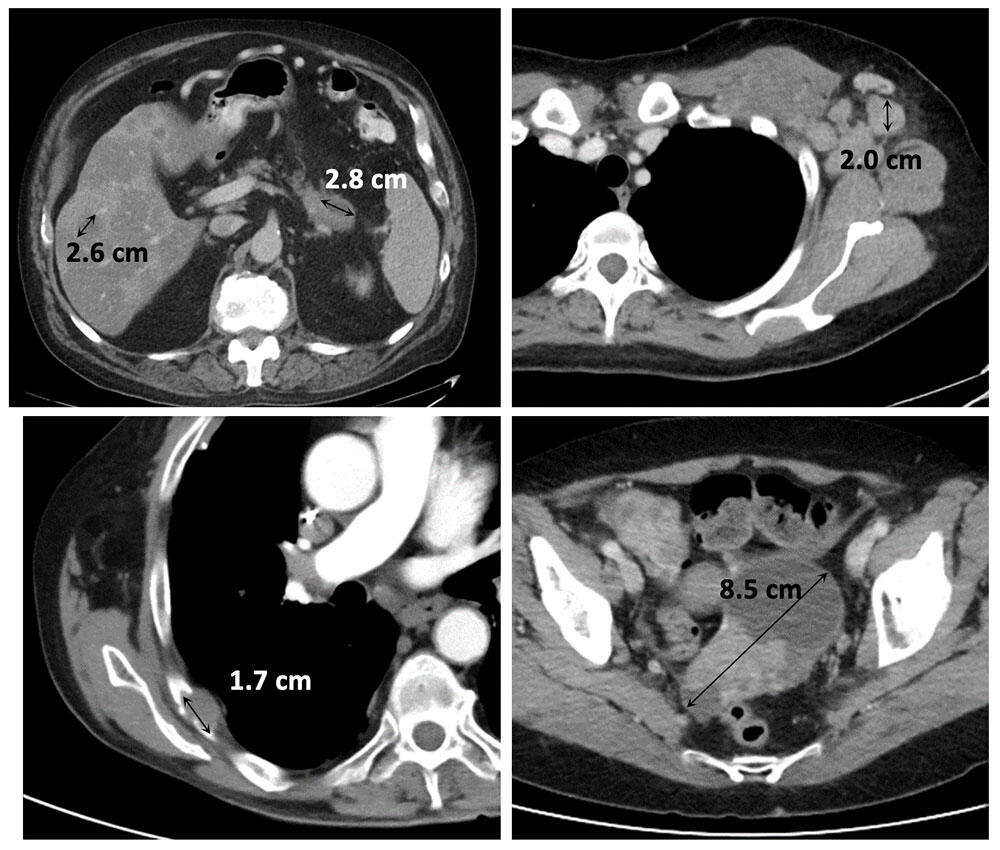 |
Non-Target Lesions
|
 |
Complete Response (CR)
- Disappearance of all targets
- Disappearance of all non-target disease
- Pathological nodes <10 mm in short axis
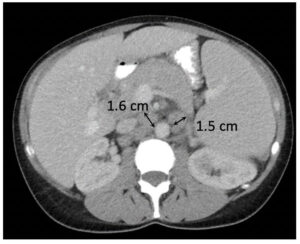
Partial Response (PR)
|
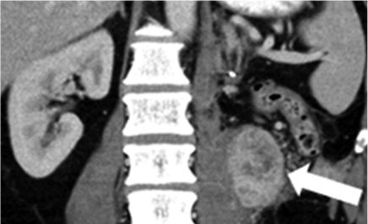
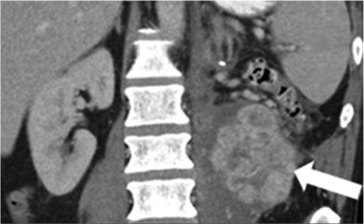
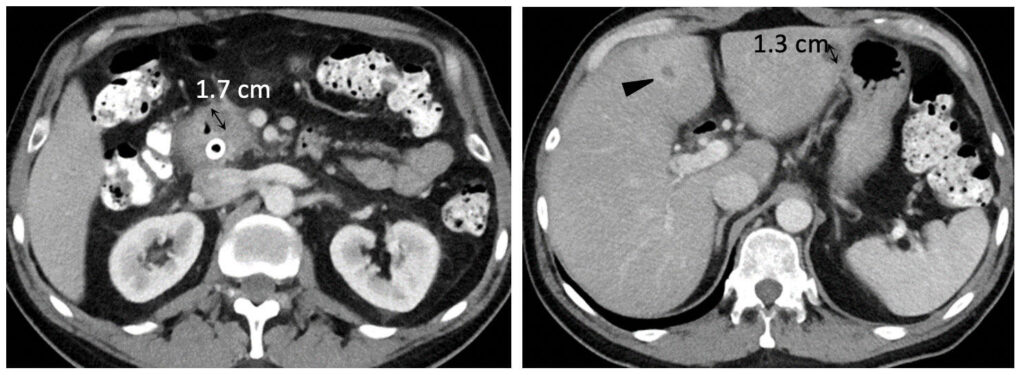 Metastatic pancreatic cancer (baseline) 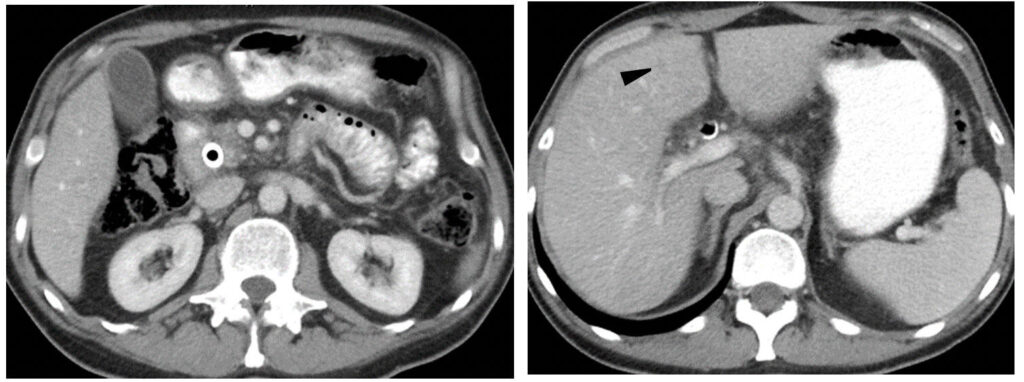 Metastatic pancreatic cancer (follow-up with deep PR) |
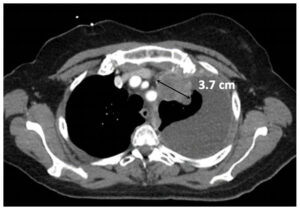
Stable Disease (SD)
|
|
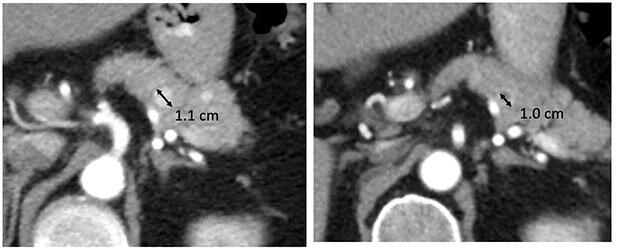
Progressive Disease (PD)
|
|
Choi H, Charnsangavej C, Faria SC et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007 May 1;25(13):1753-9.
Malignancies which may be assessed by this method
- Gastrointestinal Stromal Tumor (GIST) treated with imatinib, sunitinib, regorafenib
- Other sarcomas
- Renal cell carcinoma treated with Vascular Endothelial Growth Factor (VEGF) targeted therapy
Tumor Measurements
- Longest cross-sectional dimension of each lesion; sum long axis diameter calculated
- CT density of each tumor measured by drawing a region of interest around the margin of the entire tumor; mean HU calculated for each patient
Complete Response (CR)
- Disappearance of all lesions
- No new lesions
Partial Response (PR)
|
|
Stable Disease (SD)
- Does not meet CR, PR, PD
Progressive Disease (PD)
|
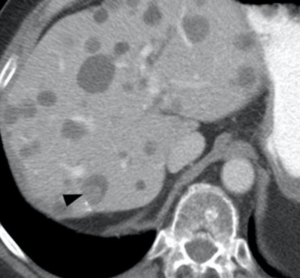
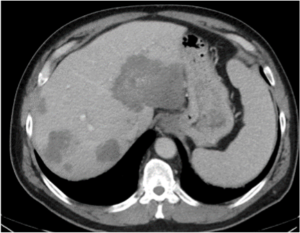
 Metastatic GIST (baseline)
|
Pitfalls
|
 Metastatic GIST (baseline)  GIST treated with sunitinib; right hepatic lesion likely demonstrates intratumoral hemorrhage with a fluid-fluid level, a potential pitfall not to be mistaken for increasing disease |
Other Criteria
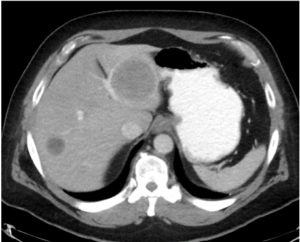
Partial Response (PR): according to CT morphologic criteria
|
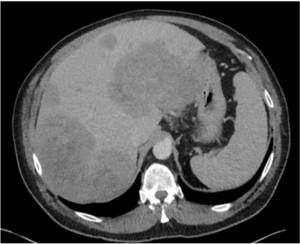 Metastatic colon cancer (baseline) |
- “I” indicates immune responses assigned using iRECIST
- Seymour L, Bogaerts J, Perrone A et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017 Mar; 18(3): e143–e152.
Target Lesion Selection and Measurement
- No change from RECIST 1.1 in target and nontarget definitions, numbers and sites of disease
Unique Features of Immune Response Assessment
New lesions
- are recorded separately and not included in the sum diameter of lesions
- result in iUPD
- on subsequent assessment after iUPD, additional new lesions or an increased size of previously new lesions (at least 5 mm for sum of new target lesion or any increase in new non-target lesion), results in iCPD
Confirmation of Progressive Disease
- If iUPD criteria met and the patient is clinically stable, treatment often continues and 4-8 week follow-up imaging is performed to assess for transient pseudoprogression or true progression
- If iUPD criteria met on basis of progression in target or non-target disease, then 4-8 week follow-up also indicated to assess for iCPD
Complete Response (iCR)
|
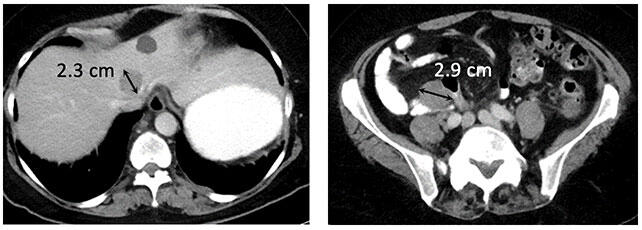 Metastatic melanoma to liver and peritoneum (baseline) 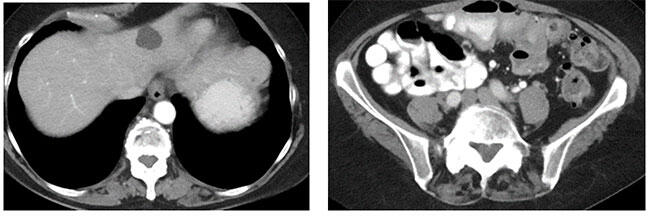 Metastatic melanoma with iCR to ipilimumab and nivolumab |
Partial Response (iPR)
|
 Metastatic melanoma (baseline) 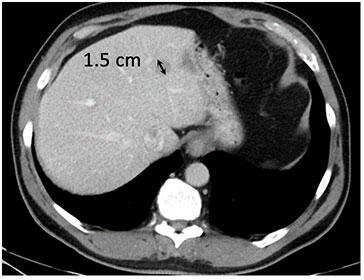 Metastatic melanoma with iPR to ipilimumab and nivolumab |
Stable Disease (iSD)
- Neither PR nor PD
- Persistence of 1 or more non-targets (non-iCR/non-iUPD)
- No new lesion
Unconfirmed Progression (iUPD)
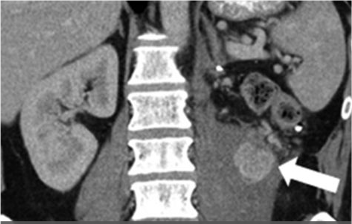
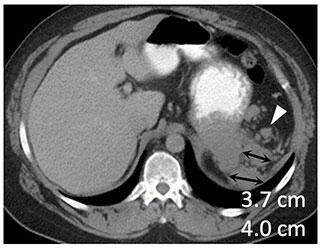
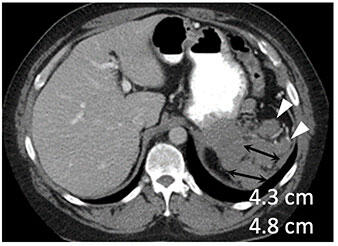
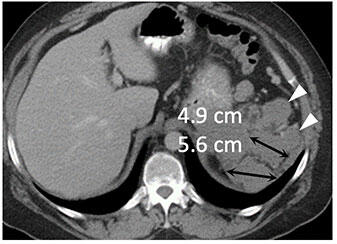
|
Metastatic renal cell carcinoma (A) treated with an immune checkpoint inhibitor, with increased L retroperitoneal disease on first follow-up resulting in iUPD (B) and decreased disease on second follow-up (C), resulting in subsequent iPR. Figure from Cancer Immunol Res. 2016 Jan;4(1):12-7. |
Confirmed Progression (iCPD)
|
Metastatic renal cell carcinoma (A) treated with combination therapy including an immune checkpoint inhibitor, with increased LUQ peritoneal disease on first follow-up (B) and further increased disease on second follow-up (C), confirming progression (iCPD). |
- Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228-47.
- Choi H, Charnsangavej C, Faria SC et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007 May 1;25(13):1753-9.
- Chun YS Vauthey J, Boonsirikamchai P et al. Association of Computed Tomography Morphologic Criteria With Pathologic Response and Survival in Patients Treated With Bevacizumab for Colorectal Liver Metastases. JAMA. 2009; 302(21):2338-2344.
- Seymour L, Bogaerts J, Perrone A et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017 Mar; 18(3): e143–e152.